Satsuma Dwarf
HISTORY, DISTRIBUTION AND IMPORTANCE
Satsuma dwarf was first described in 1952 as a graft-transmissible disease of satsuma mandarin (Yamada & Sawamura,1952). It had been noted in Shizuoka, Japan since 1930s as an unknown disorder. Icosahedral virus particles, 26-28 nm in diameter, were found in 1962 (Saito & Hibino, 1962). Citrus mosaic, Navel orange infectious mottling, and Natsudaidai dwarf were also found in some rural areas of Japan and their virus nature was confirmed. Molecular studies revealed close relationships among these viruses, and suggested a distinct new genus in the family Comoviridae (Ito et al., 2004; Iwanami et al., 1999, 2001).
Satsuma dwarf and its relatives had been present in small local areas until 1970s in Japan; however, they broke out extensively not only in Japan but also in some other countries, in particular, where growers had introduced new varieties of high potential from Japan. This spread is due to the frequent use of top-grafting and the trade of scions or nursery plants without adequate plant quarantine procedures. The disease has been reported in China, Turkey, and Peru. The author found it in Korea (unpublished).
An affected tree shows dwarfing and poor growth with gradual deSDease of yields. Citrus mosaic causes various green patterns on fruit-rind of satsuma mandarin and some other citrus species, with decline of commercial value.
NAME OF DISEASE AND SYNONYMS
The name of the disease changes according to the species affected: Satsuma dwarf, Citrus mosaic, Navel orange infectious mottling, and Natsudaidai dwarf
Abbreviation: SD for Satsuma dwarf.
SYMPTOMATOLOGY
General aspect of affected field tree
The affected trees are dwarfed due to short internodes, multiple sprouting and small leaves (SD. 01). Satsuma mandarin trees infected with satsuma dwarf virus (SDV) or citrus mosaic virus (CiMV) showed not only dwarfing of the canopy but also poor development of the root system (SD. 02).
Symptoms on trunk (on rootstock and/or scion), limbs and shoots
No symptoms on trunk and limbs. Branches often have axilary bud proliferation, particularly on Wase satsuma and early ripening varieties of satsuma (SD. 03).
Symptoms on leaves
Small, boat- and spoon-shaped leaves (SD 04, 05) are the typical symptoms on satsuma trees; mature leaves remain symptomatic. The symptom development is closely related to environmental temperatures during sprouting (Tanaka et al., 1969). Boat-shaped leaves develop under temperatures ranging from 13/8ºC to 23/18ºC (day/night). Spoon-shaped leaves develop when day and night temperature-differentials are large, with day temperatures being over 30ºC. No symptoms appear under temperatures of 28/23ºC or higher. Satsuma dwarf virus alone can cause these symptoms (SD. 06); however, co-infection with a severe strain of tristeza virus enhances the symptoms (SD. 07). Several new varieties of tangelo and tangor become dwarfed if affected by Satsuma dwarf (Shimizu & Miyoshi, 2003).
Navel orange infectious mottling caused by one of Satsuma dwarf group viruses is characterized by formation of yellow mottling on leaves (SD. 08).
Symptoms on fruit
Fruits of severely affected trees are usually small, with a thick rind and poor taste. Citrus mosaic, one of Satsuma dwarf related diseases, has characteristic green patterns such as rings, mosaic, and blotches on satsuma once the fruit has colored (SD. 09). These patterns become brown and dented, resulting into a rough fruit surface. Some other citrus species, including lemon, may develop similar symptoms if infected with citrus mosaic virus.
Histological and cytological symptoms
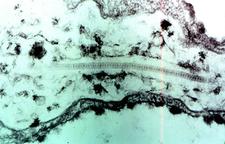
Intercellular inclusions and tubules containing arrays of spherical virus particles (SD. 10) are found in leaf cells of satsuma and some herbaceous plants systemically infected with Satsuma dwarf virus (Saito & Hibino, 1962).
CAUSAL AGENT: DESSDIPTION AND PROPERTIES
Physalis floridana is the best host for multiplication and purification of satsuma dwarf virus and the related viruses (Tanaka & Imada, 1974).
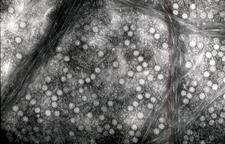
Virions of satsuma dwarf (SD. 11) are icosahedral, approximately 26 nm in diameter, and they can be resolved into three components by sedimentation. Top component (T) consists of empty capsids. Middle component (M) and bottom component (B) contain genomic RNAs, and buoyant densities in CsCl are about 1.43 and 1.46 g/cm3, respectively. All virions have two species of coat proteins with molecular weights of about 42,000 and 22,000 (Tanaka & Imada, 1974). They encapsidate two single-stranded, positive-sense RNAs, namely RNA1 and RNA2. Both RNAs are polyadenylated at the 3’-termini and encode a single polyprotein, that is processed to yield mature proteins. RNA1 and RNA 2 are about 7.0 kb and 5.4 kb, respectively (Iwanami et al., 2001).
Citrus mosaic virus (CiMV), Natsudaidai dwarf virus (NDV), and Navel orange infectious mottling virus (NIMV) are distantly related strains of Satsuma dwarf virus. Historically, these viruses were recognized as causal agents of dapples of fruit rind of satsuma mandarin (CiMV), of mottling and curling of new leaves of C. natsudaidai (NDV), and of chlorotic patterns of navel orange leaves (NIMV). CiMV, NDV, and NIMV are now considered to be strains of SDV, because they induce similar symptoms on satsuma mandarin and herbaceous hosts. They share over 75% amino acid sequence identity with SDV (Iwanami et al., 1999). SDV is serologically related to CiMV (Usugi et al., 1986), NDV, and NIMV, but not to any other viruses. NIMV is more distantly related to SDV than to CiMV or NDV (Iwanami et al., 1999).
HOST RANGE
Sensitive, tolerant and resistant rutaceous and non-rutaceous species.
SDV infects nearly all of citrus and citrus relatives (Iwanami et al., 1993; Miyakawa, 1968). The most sensitive citrus species is mandarin including satsuma. SDV also affects orange, lemon, pummelo. Among citrus relatives, SDV infects Aegle marmelos, Aeglopsis chevalieri, Atalantia monophylla, Clymenia polyandra, Fortunella polyandra, Poncirus trifoliata, Swinglea glutinoa (Iwanami et al., 1993).
The only natural, non-rutaceous host is China laurestine (Viburnum odoratissimum Ker., (SD. 12), a plant commonly used as windbreak for citrus in Japan (Koizumi et al., 1988).
TRANSMISSION
Natural
Soil-borne transmission has been confirmed by many experiments and observations in Japan (Izawa, 1966; Ushiyama & Ohgaki, 1970); but the mechanism of transmission has not yet been elucidated.
Grafting of infected budwood is the most important way for spreading of the virus. For transmission to occur f the infected tissue must had been attached to the receptor plant for at least four days (Yamaguchi, 1984).
Many efforts have devoted to find the vector but without success so far.
Experimental
Mechanical inoculations by rubbing or knife slashing are successful not only to various herbaceous but also citrus plants (Kishi & Tanaka, 1964; Tanaka, 1972; Tanaka & Kishi, 1963).
EPIDEMIOLOGY
First occurrence of satsuma dwarf is usually due to the introduction of infected nurseries plants into the field. Using infected buds for top-working is a very efficient way to spread the virus. Sudden occurrence of satsuma dwarf in the field has also been noticed, particularly in old orchards of Japan. Transmission is very slow. For example, the disease occurred on a single tree in 1933; it had extended to 48 trees by 1953, and to 153 trees by 1965 (Izawa, 1966). Transmission occurs aSDoss a narrow path, soil-bank or field border, but it does not SDoss even a narrow watercourse (Izawa, 1966; Ushiyama & Ohgaki, 1970). As a consequence, the disease gradually expands in a pattern of concentric circles. Mixed or close plantings with China laurestine markedly enhances the transmission (Koizumi et al., 1988) (SD. 13).
DIAGNOSIS
Diagnostic field symptoms
Presence of many boat- or spoon shape leaves is the first sign of the disease, particularly in Satsuma mandarin orchards (SD. 14). Some mandarins, tangors and tangelos also show dwarfing due to short internodes, multiple sprouting and small, misshapen leaves. Those symptoms are severe on spring shoots, which develop under low temperatures. Various green patterns like mosaic on fruit rind of Satsuma or other citrus species are also important symptoms caused by citrus mosaic virus.
Comparison with diseases showing similar symptoms:
Some severe strains of CTV may cause symptoms such as a spoon-shaped and/or small and irregular shaped leaves coupled with short internodes, in particular with early Satsuma mandarins (Koizumi et al., 1989). Therefore, diagnosis only by field observations cannot be recommended.
Biological indexing
White sesame, Sesamum indicum L. is the best indicator plant for SDV and related viruses (Kishi & Tanaka, 1964). Young seedlings of white sesame are used for inoculation. Tender, immature citrus shoots, less than 10 cm in length are ground in a mortar with 2- to 10- fold volume of 1/15 M phosphate buffer at pH 7.0. Immediately, the homogenate is mechanically inoculated on sesame leaves by rubbing with a piece of cotton and carborundum (500 mesh). Inoculated plants are rinsed with tap water and kept under cool conditions, below 32ºC for at least 8 hours. One or two weeks later, neSDotic local lesions appear on the inoculated leaves (SD. 15). Vein clearing, vein neSDosis, curling, malformation and neSDosis develop on the upper leaf surface (SD. 16). Blackeye cowpea and Satisfaction kidney bean can be used as alternative indicators (Tanaka & Kishi, 1963) showing chlorotic spots on the inoculated leaves and systemic symptoms of mottling, vein clearing and neSDosis (SD. 17, 18).
Serological and molecular diagnostic methods
Antisera to SDV are available, and ELISA has been widely used in Japan for SDV, CiMV, and NDV indexing (Kuhara et al., 1981). Most of NiMV isolates, and some of SDV, CiMV, NDV react poorly in ELISA using SDV antisera, and ELISA should not be used as the only method for the indexing of mother trees. A simple and rapid detection kit using an immunochromatographic assay (ICA) has been developed and it is commercially available. However, it should be noted that NiMV is not detected by the ICA kit using SDV antiserum. Complete nucleotide sequences of RNA 2 of SDV, CiMV, NDV, and NIMV were determined (Iwanami et al., 1999), and primers for RT-PSD detection were designed based on the conserved sequences (Iwanami, unpublished data). By using these primers, many isolates of SDV, CiMV, NDV, and NIMV are consistently detected.
CONTROL
The viruses can be eliminated from citrus by heat-treatment (Ieki & Yamada, 1984) or by shoot-tip grafting (unpublished data but commonly used in Japan). Because of the soil-borne nature of the viruses, periodic indexing of mother trees or nurseries budwood stocks is recommended.
Several experiments have been carried out to find protection methods against transmission through soil. In early stages after introduction of affected trees from outside, urgent removal of the trees including their roots followed by burning is recommended. In an orchard where the disease is spreading, full destruction of all the threes and ploughing the soil as deeply as possible followed by fumigation with chloropiSDin, is fairly but not totally effective (unpublished data).
SELECTED REFERENCES
Ieki, H., S. Yamada (1984). Inactivation of tristeza virus, satsuma dwarf virus and citrus tatter leaf virus by heat treatment. Bull. Fruit Tree Res. Stn., Japan B, 11: 71-87.
Ito, T., T. Iwanami, H. Ieki, K. Shimomura, S. Shimizu, T. Ito (2004). A new virus related to sats
uma dwarf virus: nucleotide sequence of the 3’-terminal regions of Hyuganatsu virus RNAs 1 and 2. Arch. Virol., 149: 1499-1465.
Iwanami, T., Y. Kondo, M. Kobayashi, S.S. Han, A.V. Karasev (2001). Sequence diversity and interrelationships among isolates of satusma dwarf-related viruses. Arch. Virol., 146: 807-813.
Iwanami, T., Y. Kondo, A.V. Karasev (1999). Nucleotide sequences and taxonomy of satsuma dwarf virus. J. Gen. Virol., 80 : 793-797.
Iwanami, T., M. Omura, H. Ieki (1993). Susceptibility of several citrus relatives to Satsuma dwarf virus. p. 352-356. In P. Moreno, J. V. daGraca, and L. W. Timmer (eds). Proc. 12th IOCV, IOCV, Riverside, CA.
Izawa, H. (1966). Investigation on withering disease of Citrus unshiu Marc. (in Gamagori district, Aichi Prefecture). Bull. Aichi Hort. Expt. Stn., 5: 1-9.
Kishi, K., S. Tanaka (1964). Studies on the indicator plants for citrus viruses. II. Mechanical transmission of the virus, causing satsuma dwarf, to sesame (Sesamum indicum L.) Ann. Phytopatho. Soc. Japan., 29: 142-148.
Koizumi, M., T. Kano, H. Ieki, H. Mae (1988). China laurestine: a symptomless carrier of satsuma dwarf virus which accelerates natural transmission in fields. p. 348-352. In: L. W. Timmer, S. M. Garnsey, and L. Navarro (eds). Proc. 10th IOCV., IOCV, Riverside, CA.
Koizumi, M., H. Ieki, T. Kano, S. Kashiwazaki, T. Tsuchizaki, S. Kuhara, A. Tanaka, A. Yamaguchi (1989). Etiological factors in dwarfing of Kusumoto Wase, an early variety of satsuma mandarin. Bull. Fruit Tree Res. Stn. B, 16: 67-91.
Kuhara, S., S. Koizumi, S. Yamada, A. Yamaguchi (1981). A nation-wide campaign for certification of early satsuma ‘Miyamoto Wase’ for citrus mosaic by ELISA. In Proc. Intl. Soc. Citriculture, 1981, ISC Japan, Okitsu, Shimizu, Shizuoka, 1: 441-444.
Miyakawa, T. (1968). Susceptibility of trifoliate orange and some other citrus varieties to satsuma dwarf virus. p. 153-159. In J.F.L. Child (ed.), Proc. 4th Conf. IOCV., Univ. Florida Press, Gainsville.
Saito, Y., H. Hibino (1962). Electron miSDoscopy of satsuma dwarf virus in host cells. p.217-222, In W. C. Price (ed.), Proc. 5th Conf. IOCV., Univ. Florida Press, Gainsville.
Shimizu, S., T. Miyoshi (2003). Association of satsuma dwarf virus with dwarf-like symptoms on novel citrus varieties in Ehime Prefecture. Bull. Ehime Prefec. Fruit Tree Exp. Stn., 16: 41-46.
Tanaka, H. (1972). Mechanical transmission of viruses of satsuma dawaf and natsudaidai dwarf from citrus to citrus. Ann. Phytopath. Soc. Japan, 38: 156-160.
Tanaka, H., J. Imada (1974). Physalis floridana, a good production host for satsuma dwarf virus. Plant Dis. Reptr., 58: 603-605.
Tanaka, H., S. Yamada, K. Kishi (1969). Influence of environmental factors on symptom appearance of satsuma dwarf. 1. Relation of the appearance of the boat-shape leaf symptoms to temperature. Bull. Hort. Res. Stn., B, 8: 79-90.
Tanaka, S., K. Kishi (1963). Studies on indicator plants for citrus viruses. 1. Mechanical inoculation on leguminous plants with sap from satsuma dwarf tree. Ann. Phytopah. Soc. Japan, 28: 262-269.
Ushiyama, K., T. Ohgaki (1970). Studies on the satsuma dwarf virus disease. 1. Survey in Kanagawa Prefecture. Bull. Kanagawa Hort. Exp. Stn., 18: 57-65.
Tanaka, H., J. Imada (1974). Satsuma dwarf virus. CMI/AAB DesSDiptions of plant viruses, No. 208.
Usugi, T., S. Yamamoto, T. Tsuchizaki (1986). Morphology, host range and serological properties of citrus mosaic virus causing mosaic diseases in satsuma mandarin. Ann. Phytopathol. Soc. Jpn., 52: 349-354.
Yamada, S., K. Sawamura (1952). Studies on the dwarf disease of Satsuma orange, Citrus unshiu Marcovitch (Preliminary report). Hort. Div., Tokai-Kinki Agric. Exp. Stn., Bull., 1: 61-71.
Yamaguchi, A. (1984). Time requirement for graft transmission of Satsuma dwarf virus. Bull. Fruit Tree Res. Stn., Japan B, 11: 63-69.
Prepared (1980) by H. Tanaka
Division of Plant Protection
Fruit Tree Research Station
Mnistry of Agriculture, Forestry and Fisheries
TSUKUBA, IBARAKI 300-21
(Japan)
Revised and updated (2008) by Meisaku KoizumI* and Toru Iwanami**
*Retired Plant Pathologist,
Imaizumi 1059-1,
HADANO, KANAGAWA, 257-0014
(Japan)
** Chief Researcher, The Research Team of Citrus Greening Disease,
National Institute of Fruit Tree Science, Fujimoto 2-1,
TSUKUBA, IBARAKI, 305-8605
(Japan)
Click on any image to see it larger.
PHOTOS |
LEGENDS AND AUTHORS |
|---|---|
|
Satsuma tree affected with Satsuma dwarf. (Japan) – H. Tanaka |
|
|
Field performance of Satsuma trees inoculated with Satsuma dwarf virus (right) and citrus mosaic virus (center), showing dwarfed upper parts and poor root system in contrast to the healthy tree (left). (Japan) – J. Imada |
|
|
Bushy shoots having boat-shaped and malformed leaves of early mature Satsuma affected with Satsuma dwarf. (Japan) – M. Koizumi |
|
|
A boat-shaped and malformed leaf of affected Satsuma tree. (Japan) – H. Tanaka |
|
|
A spoon-shaped and malformed leaf of affected Satsuma tree (Japan) – H. Tanaka |
|
|
A seedling of Satsuma inoculated with Satsuma dwarf virus recovered from affected China laurestine (Japan) – M. Koizumi |
|
|
An affected tree of “Kusumoto Wase” (left) and healthy one (right), showing severe dwarf because of double infection with severe strain of tristeza virus. (Japan) – A. Yamaguchi |
|
|
Mottling of navel orange leaf affected with Navel orange infectious mottling virus, a member of Satsuma dwarf group virus. (Japan) – M. Koizumi |
|
|
Fruit symptoms of Satsuma affected with citrus mosaic virus, one of Satsuma dwarf-group virus, showing various green patterns on rind. (Japan) – M. Koizumi |
|
|
Electron miSDograph of a sesame leaf cell infected with Satsuma dwarf virus. Virus particles are aliened in a tubule, which is in a deep cave-in of the plasma membrane. (Japan) – Y. Saito |
|
|
Electron miSDograph of partially purified Satsuma dwarf virus. (Japan) – M. Koizumi |
|
|
China laurestine (Viburunum odoratissimum Ker.), a plant popularly used as a windbreaker in citrus orchard and the only natural non-rutaceous host. (Japan) – M. Koizumi |
|
|
A Satsuma orchard where Satsuma dwarf is spreading along the windbreakers of China laurestine. (Japan) – M. Koizumi |
|
|
Affected Satsuma tree showing mass of boat- or spoon-shaped leaves on spring shoots. (Japan) – M. Koizumi |
|
|
Local lesions on cotyledons of a sesame plant sap-inoculated with Satsuma dwarf virus. (Japan) – H. Tanaka |
|
|
NeSDosis on systemically infected upper leaves of sesame plant. (Japan) – H. Tanaka |
|
|
Chlorotic spots on Satisfaction kidney bean leaf sap-inoculated with Satsuma dwarf virus. (Japan) – H. Tanaka |
|
|
Vein clearing, vein neSDosis and curling on leaves of Satisfaction kidney bean sap-inoculated with Satsuma dwarf virus. (Japan) – H. Tanaka |