Leprosis
HISTORY, DISTRIBUTION AND IMPORTANCE
Symptoms of citrus leprosis disease were first described in Florida on sweet oranges (Hume, 1901; Fawcett, 1911) but the disease has not been reported again in the USA since the 1960’ (Childers et al., 2003c).
Presently, it is endemic in South America (Argentina, Paraguay, Brazil, Bolivia, Colombia and Venezuela) as well as in Central America (Panama, Costa Rica, Guatemala and Honduras), and recently it was detected in Southern México (Rodrigues et al., 2003; Bastianel et al., 2006).
The disease is extremely destructive if mite control is not applied. In the State of São Paulo, Brazil, up to US$ 80 million are spent each year in products for chemical sprays to control the mite vector Brevipalpus phoenicis (Dragone et al., 2003). Losses are due to increase in cost of production, reduced yield, fruit drop, lower commercial value of spotted fruits, and decline/death of the trees leading to a truncated lifetime for the orchard (Rodrigues et al., 2002b).
NAME OF DISEASE AND SYNONYMS
Leprosis from lepra = ulcers (Knorr & DuCharme, 1951). Originally it was referred to as scaly bark and nail-head rust in Florida (Fawcett, 1911), “lepra explosiva” in Argentina (Frezzi, 1940), and “leprose” and “varíola” in Brazil (Bitancourt, 1955).
It is currently known that two different virus types cause leprosis disease, the prevalent cytoplasmic type (CiLV-C) and the rare, nuclear type (CiLV-N).
Abbreviation: L for leprosis; CiLV for Citrus leprosis virus
SYMPTOMATOLOGY
General aspect of affected field trees
In early infection there is premature defoliation in the interior of the canopy. Under heavier infection, intense defoliation and fruit drop occur. Advanced infection on untreated plants results in severe die-back followed by death (L. 01). A scale of severity was developed and validated for whole plant assessment (Rodrigues et al., 2002a).
Symptoms on trunk, limbs and shoots
The main trunk usually does not have lesions except when infections occur in young plants during the earlier stages of the orchard establishment. Young stems may show early chlorotic lesions, which evolve to brownish and then corky lesions (L. 02). Lesions may fuse and kill the stem by girdling and thus interrupting the phloem around the entire circumference of the stem.
Symptoms on leaves
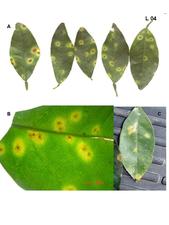
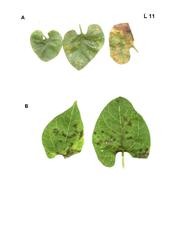
Initial symptoms caused by CiLV-C are small chlorotic spots (1-2 mm in diameter), sometimes with a necrotic center (L. 03 A), which then enlarge into spots or ring-spots of 2-3 cm of light green or yellowish color. Rings are comprised of brownish gummy cells. Sometimes the lesion follows the midrib and secondary veins. A single leaf may present several lesions (L. 03 B-D). Affected leaves tend to drop early, particularly when petioles present lesions. Leaf lesions caused by CiLV-N tend to be smaller and present a bright yellow color, rarely producing rings (L. 04). In both cases, when the leaves eventually become senescent, the area of the lesion remains greenish in contrast to the chlorotic background (L. 03 E). The degree of susceptibility for different citrus varieties plays an important role in the lesion size and consequently on lesion morphology (Rodrigues, 2000).
Symptoms on the fruits
On green fruits, either in sweet orange (L. 05 A) or mandarins (L. 05 B) scattered small chlorotic spots (2-3 mm in diameter) are initially visible. In mature fruits these lesions are larger, 5-10 mm in diameter, and acquire a brownish color with a depressed center (L. 05 C-E). Usually fruits with lesions fall prematurely. Both CiLV-C and CiLV-N (L. 05 F) cause similar fruit symptoms. The lesions affect only the external part of the fruit and do not reach the albedo.
Histological and cytological symptoms
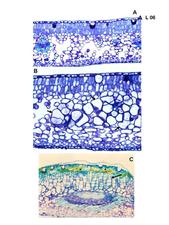
Anatomy of leaf, fruit and stem lesions reveals the presence of hypertrophied spongy parenchyma cells and hyperplastic palisade parenchymal cells interspersed with necrotic cells in advanced lesions (Marques et al., 2004) (L. 06). A clear reduction in starch accumulation in the epidermis of sweet orange leaves and stems lesions occurs, which was corroborated by transcript analyses of CiLV-C infected sweet orange (J. Freitas-Astua, unpublished data).
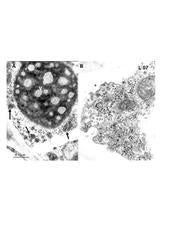
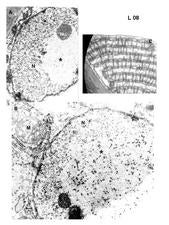
In ultrathin sections of lesions caused by CiLV-C, virions within membrane-bound vesicles and/or electron dense, circular to elliptical, vacuolated viroplasms, usually single, are present in the cytoplasm (Colariccio et al., 1995) (L. 07). In cells infected with CiLV-N a characteristic nuclear, electron lucent viroplasm is detected as well as short rod-like particles in the nucleus or cytoplasm (Kitajima et al., 1972) (L. 08). In early lesions these cytopathic effects are visible in most of the parenchymal and epidermal cells, but in old lesions they are observed in very few cells. These alterations are usually absent from the vascular parenchyma (Rodrigues et al., 2003).
CAUSAL AGENT: DESCRIPTION AND PROPERTIES
It is generally accepted that the leprosis disease of citrus is caused by either CiLV-C or CiLV-N, both transmitted by Brevipalpus mites (Rodrigues et al., 2003). No case of co-infection has yet been reported. The complete CiLV-C genome has been sequenced. It consists of two segments (5 and 9 kb) of positive sense ssRNA with poly-A tail (Pascon et al., 2006; Locali-Fabris et al., 2006) (L. 09). Its unique genomic sequence, structure, and organization led Locali-Fabris et al. (2006) to propose CiLV-C as the prototype of the Cilevirus, a new virus genus. The particles are bacilliform, 50-70 nm wide x 120-130 nm long, and always present in membrane bound cavities in the cytoplasm. Little is known about the CiLV-N genome. It may belong to the same group as Orchid fleck virus (OFV), also a B. californicus-transmitted virus with which it shares cytopathology and virion morphology. OFV is a bipartite rhabdovirus(proposed genus- Dichorhabdovirus) and its particle is short, rod-like, and 40-50 nm x 100-110 nm (Kondo et al., 2006). CiLV-N has been found mainly in backyard orchards and seems to prefer cooler places.
HOST RANGE
Sweet orange and mandarins are the most common plants naturally infected by CiLV-C or CiLV-N. A non-citrusrutaceous plant (Swinglea glutinosa (Blanco)Merr) was found naturally infected by CiLV-C in Colombia (G.E. Leon, J. Freitas-Astua & E.W. Kitajima, unpublished data) (L. 10). Experimental infection of CiLV-C was achieved by mechanical means to herbaceous hosts (Chenopodium quinoa Wild., C. amaranticolor Coste & Reyn. and Gomphrena globosa L.) (Colaricio et al., 1995) (L. 11). CiLV-C was also experimentally mite-transmitted to, Solanum violaefolium Schott (Rodrigues et al., 2005), Phaseolus vulgaris L. (Groot et al., 2006) (L. 12) Malvaviscus arboreus Cav., Hibiscus rosa-sinensis L., Grevilea robusta A. Cunn., Bixa orellana L. and Commelina benghalensis L. (Nunes et al., 2006) (L. 13). No other host plant besides sweet oranges and mandarins is known for CiLV-N.
Sensitive species, varieties or combinations
All sweet orange varieties are susceptible. Mandarins, grapefruit and some tangors present variable degrees of resistance. Lemons and limes are considered immune to leprosis (Bitancourt, 1955; Rodrigues, 2000; Rodrigues et al., 2003; Bastianel et al., 2006).
Symptomless species, varieties or combinations
None.
TRANSMISSION
Natural
Both CiLV-C and CiLV-N are spread by the mite vector Brevipalpus (Acari: Tenuipalpidae). B. phoenicis (L. 14, 15)is the main vector species, but there are reports of leprosis symptoms association with B. obovatus and B. californicus (Vergani, 1945; Knorr, 1968). There is no report of vertical transmission of the viruses in viruliferous mites. Brevipalpus mites are polyphagous and present in tropical and subtropical regions in the world (Vergani, 1945; Rodrigues et al., 2003; Childers et al., 2003b).
Experimental
B. phoenicis acquires the virus upon feeding on leprosis lesions and transmits it to healthy citrusplants. All developmental stages of the mite are able to transmit the virus. Under experimental conditions B. phoenicis transmitted CiLV-C to other non-citrus plants (see host range). CiLV-C was transmitted mechanically from citrus to citrus and to some herbaceous plants (see host range)causing local lesions (Colariccio et al., 1995).
Patch grafting of stem or leaf lesions onto healthy stems caused the lesions to expand to the healthy tissues (Knorr, 1968).
EPIDEMIOLOGY
Both CiLV-C and CiLV-N cause only localized infection, not systemic, in citrus. Thus, mites that feed on the lesions and become viruliferous, are necessary to disseminate the virus. Brevipalpus mites are slow moving and usually look for shelter in surface cracks and wounds. In commercial orange blocks, work by Bassanezzi & Laranjeiras (2007) indicated the lack of spatial association between mite (Brevipalpus phoenicis)-infested plants and citrus leprosis-affected plants. The time lag between the mite infestation and the symptom development as well as the disrupting effect caused by frequent acaricide sprays may cause this non-overlapping pattern. Epidemiological curves made in the State of São Paulo indicate that the highest severity is achieved in dry months (Rodrigues et al., 2003). Leprosis epidemics usually start with the introduction of viruliferous mites from infected orchards nearby, the mites being carried by asymptomatic rooted plants, tools, boxes and/or worker’s clothes.
DIAGNOSIS
Diagnostic field symptoms
Localized lesions on leaf, stem or fruit associated with Brevipalpus mite infestation occur mainly on sweet orange and mandarin.
Comparison with other diseases
Leprosis lesions are very characteristic, but may sometimes be confused with those caused by bacterial canker and zonate chlorosis. Canker lesions and scaling on the twigs resemble those caused by psorosis, but leprosis scales tend to become dark colored and thicker. Heavy infestation by Brevipalpus mites causes leaf and fruit injuries that somewhat resemble leprosis symptoms (Childers et al., 2003a).
Biological indexing
Not used.
Electron microscopy
Until the advent of molecular techniques, transmission electron microscopy was a routine technique used for diagnosis, detecting virions and/or viroplasmas in the cells of the lesions. It is still useful to confirm the presence of CiLV-N (Kitajima et al., 2003).
Serological and molecular diagnostic methods
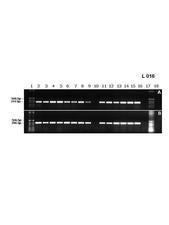
CiLV-C and CiLV-N have not yet been purified, but Manjunath et al. (2006) reported production of an antiserum against a viral protein, detectable by Western blot. Locali et al. (2003) reported the first molecular tool for CiLV-C detection by RT-PCR when they designed primer pairs for the movement protein and viral replicase genes (L. 16). Now this method is widely used to detect CiLV-C. Even though lesions from fresh leaves are better, the virus has been also detected in dried material. The method is very sensitive and can detect CiLV-C in about three viruliferous mites.
CONTROL
The best way to keep an orchard leprosis-free is to start with healthy plants from reliable nurseries. Windbreaks to slow the dispersal of the viruliferous mites by wind as well as control of entry of tools, boxes and workers are recommended. Unless an endemic infection occurs, chemical spray to control Brevipalpus is not required. If spraying is practiced one must remember that mites develop resistance to these chemicals, and a rotation of the products is recommended. Elimination of infected tissues by removing or pruning heavily infected plants helps to reduce inoculum (Rodrigues et al., 2002b, Dragone et al., 2003; Rodrigues et al., 2003; Bastianel et al., 2006). After the CiLV-C genome was sequenced, transgenic plants expressing viral genes have been produced (Alellyx Applied Genomics, personal communication) and are now under test for resistance. If successful, it may open new strategies for the control of leprosis.
SELECTED REFERENCES
Bassanezi, R.B., F.F. Laranjeira (2007). Spatial patterns of leprosis and its mite vector in commercial citrus groves in Brazil. Plant Pathology, 56, 97-106.
Bastianel, M., J. Freitas-Astua, E.W. Kitajima, M.A. Machado (2006). The citrus leprosis pathosystem. Summa Phytopathologica, 32, 211-220.
Bitancourt, A.A. (1955). Estudos sobre a leprose dos citros. Arquivos do Instituto Biológico, 22, 161-231.
Childers, C.C., J.V. French, J.C.V. Rodrigues (2003a). Brevipalpus californicus, B. obovatus, B. phoenicis and B. lewisi (Acari: Tenuipalpidae): a review of their biology, feeding injury and economic importance. Experimental and AppliedAcarology, 30, 5-28.
Childers, C.C., J.C.V. Rodrigues, W.C. Welbourn (2003b). Host plants of Brevipalpus californicus, B. obovatus and B. phoenicis (Acari: Tenuipalpidae) and their potential involvement in the spread of viral diseases vectored by these mites. Experimental and Applied Acarology, 30, 29-105.
Childers, C.C., J.C.V. Rodrigues, K.S. Derrick, D.S. Achor, J.V. French, W.C. Welbourn, R. Ochoa, E.W. KIitajima (2003c). Citrus leprosis and its status in Florida and Texas. Experimental and Applied Acarology, 30, 181-202.
Colariccio, A., O. Lovisolo, C.M. Chagas, S.R. Galletti, V.V. Rossetti, E.W. Kitajima (1995). Mechanical transmission and ultrastructural aspects of citrus leprosis disease. Fitopatologia Brasileira, 20, 208-213.
Dragone, D., Rodrigues, J.C.V., Neves, E.M., Nogueira, N.L. (2003). Viabilidade econômica do controle da leprose dos citros em variedades de laranja. Laranja, 24, 311-328.
Fawcett, H.S. (1911). Scaly bark or nail-head rust of citrus. Florida Agricultura Experimental Station,Bulletin 106, 41p.
Frezzí, M.J. (1940). La lepra explosive del naranjo. (Argentina) Ministerio de Agricultura, Boletín Frutas y Hortalizas, 5 (46), 3-16.
Groot, T.V.M., J. Freitas-Astua, E.W. Kitajima (2006). Brevipalpus phoenicis transmits Citrus leprosis virus, cytoplasmic type (CiLV-C) to common bean (Phaseolus vulgaris) Ander experimental conditions. Virus Reviews & Research, 11 (supl.), 67.
Hume, H.H. (1901). Some fungous diseases of citrus and others fruits. Proc. Florida State Hort. Soc., 14, 64-70.
Kitajima, E.W., G.W. Müller, A.S. Costa, V.A. Yuki (1972). Short, rod-like Particles associated with citrus leprosies. Virology, 50, 254-258.
Kitajima, E.W., C.M. Chagas, J.C.V. Rodrigues (2003). Brevipalpus-Transmitted plant virus and virus-like diseases: cytopathology and some recent cases. Experimental and Applied Acarology, 30, 135-160.
Knorr, L.C. (1968). Studies on the etiology of leprosis in citrus. p. 351-357. In J.F.L. Childs (ed.). Proc. 4th Conf. Intern. Organization Citrus Virol., Univ.Florida Press, Gainesville.
Knorr, L.C. & E.P. DuCharme (1951). The relationship between Argentina’s lepra explosiva and Florida s scaly bark with implications for the Florida citrus grower. Plant Disease Reporter, 35, 70-75.
Kondo, H., Maeda, T., Shirako, Y. & Tamada, T. (2006). Orchid fleck virus is a rhabdovirus with an unusual bipartite genome. Journal of General Virology, 87, 2413-2421.
Locali, E.C., J. Freitas-Astua, A.A. Souza, M.A.Takita, G. Astua-Monge, R. Antonioli, E.W. Kitajima, M.A. Machado (2003). Development of a molecular tool for the diagnosis of leprosis, a major threat to citrus production in the Americas. Plant Disease, 87, 1317-1321.
Locali-Fabris (E.C.), J. Freitas-Astua, A.A. Souza, M.A. Takita, G. Astua-Monge, R. Antonioni-Luizon, V. Rodrigues, M.L.P.N. Targon, M.A. Machado (2006). Complete nucleotide sequence, genomic organization and phylogenetic analysis of citrus leprosis virus, cytoplasmic type. Journal of General Virology, 87, 2721-2729.
Manjunath, K.L., E. Rangel, A.S. Guerra-Moreno, R.H. Brlansky, R.F. Lee. (2006) Serological detection of the cytoplasmic Citrus leprosis virus (CiLV) from Infected citrus plants. Phytopathology, 96, S 163.
Marques, J.P.R., B. Apezzatto-da-Glória, E.W. Kitajima (2004). Anatomia das lesões foliares e de frutos de Citrus spp. causadas pela doença leprose. In Congresso Brasileiro de Botânica, 55. Viçosa, Sociedade Botânica do Brasil. CD Rom.
Nunes, M.A., E.W. Kitajima, J. Freitas-Astua, M.E. Hill, T.R. Gottwald, C.A.L. (2006). Infecção de hibisco e malvavisco pelo vírus da leprose dos citros através de Brevipalpus phoenicis (Acari: Tenuipalpidae). In Resumos Simpósio Brasileiro de Acarologia I. Viçosa, Universidade Federal de Viçosa. p. 218.
Pascon, R.C., J.P. Kitajima, M.C. Breton, L. Assumpção, C. Greggio, Zanca, A.S., V.K. Okura, M.C, Alegria, M.E. Camargo, G.G.C. Silva, J.C. Cardozo, M.A.I. Vallim, S.F. Franco, V.H. Silva, H. Jordão Jr., F. Oliveira, P.F. Giachetto, F. Ferrari, C.L. Aguilar-Vildoso, F.J.B. Franchiscini, J.M.F.Silva, P. Arruda, J.A. Ferro, F. Reinach & A.C.R. Silva (2006). The complete nucleotide sequence and genomic organization of Citrus leprosis associated virus, cytoplasmic type (CiLV-C). Virus Genes, 32, 289-298.
Rodrigues, J. C. V. (2000) Relaçao patógeno-vetor-planta no sistema leprose dos citros. PhD Diss., Univ. Sao Paulo, Piracicaba, SP. 168pp.
Rodrigues, J.C.V.; Nogueira, N.L.; Machado, M.A. (2002a) Elaboração e validação de escala diagramática para leprose dos citros. Summa Phytopathologica, 28, 192-196.
Rodrigues, J.C.V.; Ueta, F. Z.; Muraro, R.P. (2002b) Opções e custos comparativos para um programa de redução do inóculo da leprose dos citros. Laranja, 23, 333-344.
Rodrigues, J.C.V., E.W. Kitajima & C.C. Childers (2003). Citrus leprosis vírus vectored by Brevipalpus phoenicis (Acari: Tenuipalpidae) on citrus in Brazil. Experimental and Applied Acarology, 30, 161-179.
Rodrigues, J. C. V. ; Locali, E. ; Freitas-Astúa, J. ; Kitajima, E. W. (2005). Transmissibility of citrus leprosis virus by Brevipalpus phoenicis in Solanum violaefolium. Plant Disease, 89, 911-911.
Vergani, A.R. (1945). Transmission y naturaleza de la lepra explosiva del naranjo. (Argentina) Ministerio Agricultura, Instituto de Sanidad Vegetal, Serie a 5 (3), 3-11.
Prepared (1975) by L.C. Knorr
Addendum (1975) by V. Rossetti
Instituto Biològico
C.P. 7119 –SAO PAULO,
(Brazil)
Revised and updated (2007) by
E.W. Kitajima & C.M. Chagas
Departamento de Entomologia, Fitopatologia e Zoologia Agrícola
ESALQ/USP
CP 9, 13418-900 Piracicaba, SP
(Brazil)
Juliana Freitas-Astua
Embrapa Milho e Sorgo/Centro APTA Citros Sylvio Moreira
CP 4
13590-000 Cordeirópolis, SP
(Brazil)
J.C.V. Rodrigues
Univ. Puerto Rico
Estacion Experimental Agrícola, Jd.Botânico Sur,
Crop Prot.Dept., Coll.Agric.Sci.,
1193 Calle Guayacan
San Juan, PR 00926
(USA)
Click on any image to see it larger.
PHOTOS |
LEGENDS AND AUTHORS |
|---|---|
|
Field ‘Pera’ sweet orange plants about 4 years old, in a leprosis-infected Orchard, Piracicaba, SP, Brazil. Left plant was kept continuously with acaricide, while the right, untreated plant, shows a severe die back.- J.C.V. Rodrigues
|
|
|
Lesions on the branches of a sweet orange stem caused by CiLV-C. A. Lesions on a stem of field Pera sweet orange (Brazil) - J. Freitas-Astua. B. Stem lesions on different degrees of development in Valencia sweet orange. (Panamá) - E.W. Kitajima. C. Detail of a relatively new lesion on the stem of Pera sweet orange (Brazil) – E.W. Kitajima
|
|
|
A composite plate showing different types and sizes of the lesions caused by CiLV-C in sweet orange leaves. A. Few-weeks old, young lesions on Pera sweet orange caused by experimental infestation with viruliferous Brevipalpus phoenicis mites. B-D. Different aspects of older lesions in sweet orange leaves, varying from chlorotic spots, chlorotic rings to large, reddish spots with concentric rings. Larger lesions usually have a brownish central spot. Sometimes lesions follow the mid or lateral veins. Number of lesions also varies from one to more than a dozen per leaf. Initial lesions are small, a few millimeters in diameter. E. Usually leaves with lesions tend to fall prematurely but when they remain and become senescent, the lesion may appear as green spots or rings against a chlorotic background. (Brazil) – E.W. Kitajima
|
|
|
Leaf lesions caused by CiLV-N in sweet orange leaves. A. Lesions are usually smaller, with a bright yellow halo surrounding a brownish central spot. Some lesions show a small brownish to reddish central spot surrounded by a ring, and then the yellow halo. (Bazil) – E.W. Kitajima. B. Detail of the lesions. (Brazil) – E.W. Kitajima. C. Same as A, but in a sample from Panama. E.W. Kitajima
|
|
|
Symptoms on fruits. A-E, lesions caused by CiLV-C and F, by CiLV-N. A and B. Small, chlorotic lesions on a sweet orange (A) (Brasil – E.W. Kitajima) and mandarin (B)(Brazil – J.C.V. Rodrigues) fruits. C. Different degrees of the lesion development in green ‘Pera’ sweet orange fruits (Brazil) – E.W. Kitajima. D. Lesions of different sizes in maturing ‘Valencia’ sweet orange fruits. (Brazil) – E.W. Kitajima E. Large, browinish and depressed lesions on a ‘Valencia’ sweet orange fruit. (Brazil – E.W. Kitajima) F. Fruit lesions in ‘Valencia’ sweet orange in a orchard, caused by CiLV-N. (Panama) – E.W. Kitajima
|
|
|
Anatomical alterations in a ‘Pera’ sweet orange leaf lesion caused by CiLV-C. A. Section of a healthy control leaf. B. Section of a leprosis lesion. Note that many spongy parenchyma cells are hypertrophied. C. A section through a small vein. Note the yellowish gum accumulation and hiperplasia in palisade parenchyma cells above the vascular region. (Brazil- J.P.R. Marques)
|
|
|
Morphology of CiLV-C and cell alterations as seen by transmission electron microscopy of the leprosis leaf lesions. A. Characteristic electron dense and vacuolated inclusions (viroplasm) in the cytoplasm of a leaf parenchyma cell. Groups of short, bacilliform, membrane-bound CiLV-C particles (60-70 nm x 110-120 nm) are present nearby, contained in the cisternae of the endoplasmic reticulum. B. Detail of a cytoplasmic area with many vesicles containing the viral particles. – E.W. Kitajima
|
|
|
Transmission electron microscopy of the leaf lesions caused by CiLV-N. A. Nucleus from a lesion in a ‘Valencia’ sweet orange leaf showing a large, electron lucent mass (viroplasm), a characteristic feature of the nuclear type of the viruses transmitted by Brevipalpus mites (Brazil). B. Similar to A, showing short, rodlike particles scattered within the viroplasm. Some of them are present in the adjacent cytoplasm, associated with elements of the endoplasmic reticulum. (Brazil). C. A peculiar arrangement of layers of rodlike viral particles perpendicularly associated with the endoplasmic reticulum (Panama). – E.W. Kitajima
|
|
|
Schematic representation of the CiLV-C bi-partite, positive sense, ssRNA genome. Functional domains attributed according to the translated sequences are indicated inside the rectangles. For those translated sequences with unidentified functions, the approximate molecular mass (in KDa) is indicated inside the rectangles. MTR = methyltransferase domain; C-Prot = cysteine protease domain; HEL = helicase domain; RdRp = RNA dependent RNA polymerase domain; MP = movement protein domain. – E. Locali-Fabris, J. Freitas-Astua & M.A. Machado
|
|
|
Chlorotic and ringspot lesions in Swinglea glutinosa, used as windbreaks around citrus orchards in Gamal, Meta, Colombia. Electron microscopy and RT-PCR demonstrated the presence of CiLV-C in the lesions. – G.E. Leon & E.W. Kitajima
|
|
|
Local lesions in Chenopodium quinoa and C. amaranticolor leaves, mechanically inoculated with extracts from leaf leprosis lesions caused by CiLV-C in Pera sweet orange (Brazil). – E.W. Kitajima
|
|
|
Local lesions in Solanum violaefolium (A) (Brazil) – J.C.V. Rodrigues & E.W. Kitjaima, and common bean (Phaseolus vulgaris) (The Netherlands) (B) – T.V.M. Groot & E.W. Kitajima, caused by experimental infestation with Brevipalpus phoenicis mites colonized on sweet orange leaf lesions caused by CiLV-C. Infection by CiLV-C was confirmed by electron microscopy and RT-PCR (Brazil).
|
|
|
Chlorotic spots and ringspots on a leaf of Hibiscus rosa-sinensis (A) and brownish spots on partially senescent Malvaviscus arboreus (B)leaves after infestation with B. phoenicis mites collected from CiLV-C infected sweet orange plants. Presence of CiLV-C in the lesions was confirmed by electron microscopy and RT-PCR. (Brazil). – M.A. Nunes & J. Freitas-Astua
|
|
|
The mite Brevipalpus phoenicis as seen under stereomicroscoscope. (A) A view of an adult female (Brazil). – P.T.O. Ferreira. (B) B. phoenicis colony on a sweet orange fruit under stereomicroscope (Brazil). – J.C.V. Rodrigues
|
|
|
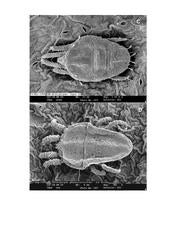
Scanning electron microscopy of a six legged larva (C) and adult (D) Brevipalpus phoenicis (Brazil) – E.W. Kitajima
|
|
|
An example of electrophoretic profile of RT-PCR products used for CiLV-C detection. Bands formed by amplified CiLV-C cDNA from extracts of fourteen different sweet orange samples symptomatic for leprosis, collected at the State of São Paulo. Lane 10 is from a healthy, uninfected plant. Lanes 1 and 18 are 1 kbp molecular markers. Bands in A and B were produced using primers for the genes that code for the viral movement protein, and the replicase, respectively (Brazil). – E. Locali, J. Freitas-Astua & M.A. Machado
|