Huanglongbing
HISTORY, DISTRIBUTION AND IMPORTANCE
History. In South China, in 1919, Reinking mentioned “yellow shoot” as a citrus disease of little importance (Reinking, 1919). By 1936, the “yellow shoot disease”, named “huang long bing” in Chinese, had spread and became a serious problem to which Kung Hsiang Lin, a phytopathologist at the South China Agricultural University, devoted most of his activities from 1941 to the late 1950s. From his discussions with the farmers in the Chaozhou district, Guangdong province, near the South China Sea (HLB. 001), he learned that the disease on which he was working, huanglongbing (HLB), and which had been seen by Reinking in 1919, had been in the Chaozhou district since the late 19th century, and that the disease in South China probably originated from there (Lin, 1956).
K. H. Lin (HLB. 002) was the first to report in 1956, after many years of research, that HLB was transmissible by graft-inoculation (Lin, 1956). Professor A. Ciccarone (HLB. 002), an Italian phytopathologist, published a report of Lin’s work in 1957 in an Italian journal of citriculture (Ciccarone, 1957), but in spite of this report, Lin’s achievements remained largely unknown in the western world. Transmission of South African greening by graft-inoculation was only reported nine years later, in 1965 (McClean and Oberholzer, 1965a). Transmission of the greening agent by the African citrus psyllid vector, Trioza erytreae, was also published in 1965 (McClean and Oberholzer, 1965b). Transmission of the HLB agent by the Asian citrus psyllid vector, Diaphorina citri, was reported simultaneously in 1967 in India (Capoor et al., 1967) and in the Philippines (Martinez and Wallace, 1967).
The disease was described in the Philippines in 1921, but thought to be related to zinc deficiency. Similarly, in South Africa the disease, known since 1928 /1929 as "yellow branch" in the western Transvaal and "greening" in the eastern Transvaal, was still assumed in 1937 to be a mineral nutrition problem (van der Merwe and Andersen, 1937).
Citrus in India has been known to suffer seriously from certain disorders resulting in low production, twig dieback, slow death, and even sudden wilting. These symptoms were attributed to “dieback”, a disease that was first observed by Roghoji Bhonsale (cited by Capoor, 1963) in the 18th century, soon after the introduction of citrus into India. It was also observed by Bonavia (1888) in Assam. However, the problem with Indian “dieback” comes from the fact that “dieback in citrus is not a specific disease” (Asana, 1958) and, over the years, many factors, including soil disorders, nutritional deficiencies, twig fungi, and viruses such as citrus tristeza virus, were evoked to account for it (Capoor, 1963). Support for the involvement of huanglongbing in dieback came in 1966 from a survey on dieback in all major citrus areas of India, which concluded that dieback was caused by the “virus” responsible for greening in South Africa (Fraser & Singh, 1968). Unquestionable proof for the presence of huanglongbing in India came in 1967 when successful transmission of the disease agent with the Asian citrus psyllid was obtained (Capoor et al., 1967).
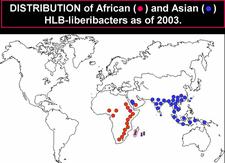
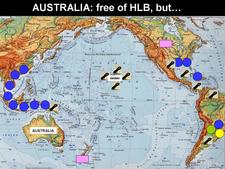
Distribution. [See Bové (2006) for references on occurrence of HLB and HLB-vectors in certain countries]. Until 2003 (HLB. 003), HLB was restricted to two large geographical regions: Africa and Asia, with the Arabian Peninsula in between.
The Asian HLB-associated liberibacter (Candidatus Liberibacter asiaticus) and the Asian psyllid vector, Diaphorina citri, were present in the following countries of Asia, Southeast Asia, and Oceania: India, Pakistan, Nepal, Bhutan, Bangladesh, Sri Lanka, Myanmar, Thailand, Malaysia, Cambodia, Laos, Vietnam, China including Taiwan, Japan (Ryukyu and Okinawa islands), Philippines, Indonesia (Java, Sumatra, Eastern Kalimantan, Southern Sulawasi, Bali), East Timor, and Papua New Guinea. In 2008, southern and southeastern Iran (HLB. 004) had to be added to the list of affected Asian countries (Hossein Taheri, personal communication). In all these countries, the HLB agent and the psyllid vector are temperature tolerant, and symptoms of Asian HLB occur at temperatures up to 32°C, if not higher. Under experimental conditions, symptoms of Asian HLB were well expressed at 32°C (Bové et al., 1974), and even 35°C (Lopes et al., 2009b).
The African HLB-associated liberibacter (Candidatus Lberibacter africanus) and the African psyllid vector were present in the following countries of Africa: South Africa, Zimbabwe, Swaziland, Malawi, Burundi, Tanzania, Kenya, Somalia, Ethiopia, Cameroon, Nigeria and Central African Republic as well as Madagascar island. In these countries, the disease and the African psyllid vector are only present in cool, elevated areas with temperatures below ~30°C, as the African HLB-associated liberibacter and the African psyllid are both temperature sensitive (Catling, 1969c). Under experimental conditions, symptoms of African HLB were well expressed at 27°C, but not at 32°C (Bové et al., 1974). The African psyllid is also severely affected by relative humidities below 25%. The combination of hot and dry conditions is most detrimental to the African vector.
In Saudi Arabia, HLB and the Asian psyllid were present in the early 1980s in the western oases along the Red Sea from Mecca down to the border with Yemen. In Yemen, HLB and the African vector occurred only on cool uplands, the HLB-associated liberibacter and its vector being both of the temperature-sensitive type. At the Saudi / Yemeni border, the two psyllid vectors were found in the same orchards, and the two HLB-associated liberibacters, the African one and the Asian one, were probably present too (Bové and Garnier, 1984)
The two psyllid vectors, T. erytreae and D. citri, as well as the two HLB-associated liberibacters, the Asian one and the African one, were present In the two Indian Ocean islands, Reunion and Mauritius (Garnier et al., 1996). In Reunion for instance, the Asian HLB-associated liberibacter and D. citri occur from sea level up to an altitude of ~500m. Above 500m, the African HLB-associated liberibacter and T. erytreae take over. In both islands, some sweet orange trees carry both the African liberibacter and the Asian liberibacter.
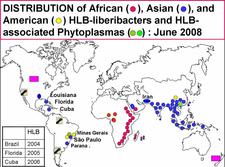
In America (HLB. 004), HLB has been recognized for the first time in the state of São Paulo (SSP), Brazil, in 2004 (Coletta-Filho et al., 2004;Teixeira et al., 2005a, b, c ,d, and e), next in 2005 in Florida, USA (Halbert, 2005), and in 2006 in Cuba (Luis Pantoja et al., 2008). In all three places, the disease was probably present, unrecognized, several years before it was reported. In Florida and Cuba, the HLB-associated liberibacter is of the Asian type. In SSP, two HLB-associated liberibacters are present, the Asian HLB bacterium and a new bacterium, the American liberibacter (Candidatus Liberibacter americanus). In 2004/2005, the American liberibacter was present in 95% of trees and the Asian liberibacter in about 5%. However, by 2008 the situation has reversed more trees being found infected with Ca. L. asiaticus than with Ca. L. americanus. This evolution might be related to the fact that Ca. L. asiaticus is heat tolerant, while Ca. L. americanus was found to be heat sensitive (Lopes et al., 2009b).The Asian psyllid vector was reported in SSP as early as 1942, but in Florida, only in 1998 and in Cuba in 1999. Lastly, HLB associated with Ca. L. asiaticus and D. citri has been identified in New Orleans (Louisiana, USA) in 2008.
Some citrus regions carry HLB psyllid vectors, but not (yet) the disease:
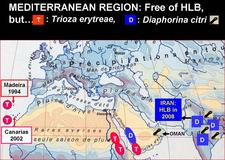
- T. erytreae is present in the islands of Saint Helena and Madeira (Portugal), as well as in four Canary islands (Spain): Tenerife, La Gomera, La Palma and El Hierro (HLB. 005).
- In South America, D. citri was found so far in Argentina in the Entre Rios region in 1994 (Vaccaro, 1994) and the North West region in 2006 (Augier et al., 2006), and in the Paraguana peninsula of Venezuela in 1999 (Cermeli et al., 2000) (HLB. 006).
- Regarding Central America, D. citri was found in Honduras (Burckhardt and Martinez, 1989), Belize (see Halbert and Nuñez, 2004), and Costa Rica (Villalobos et al., 2005).
- In the West Indies and Caribbean islands, D. citri was recorded in Guadeloupe (France) in 1998 (Etienne et al., 1998), Bahamas in 1999, Cayman islands in 2000, Virgin Islands and Dominican Republic in 2001, and Puerto Rico in 2002 (see Halbert and Nuñez, 2004). In the meantime, the Dominican Republic has become affected by HLB.
- In North America, D. citri is present in the Rio Grande Valley of Texas (USA) since 2001, and in the Yucatan peninsula of Mexico since 2002. By 2008, It was found all over Mexico, and in June 2008 it was seen in Tijuana, at the border between Baja California, Mexico, and California, USA. By the end of 2008, it was eventually seen in California, and it was present throughout the Southern U.S.A, including Texas, Alabama, Georgia, Mississippi, South Carolina, without omitting Florida and Louisiana where both D. citri and Ca. L. asiaticus are present.
- In the Pacific Ocean, the islands of Hawaii and Maui also carry D. citri (Conant et al., 2007).
- Finally, in the Arabian Peninsula, the Sultanate of Oman (HLB. 005), which has Yemen plus African HLB to the Southwest, and Iran plus Asian HLB to the North, has now been declared fully invaded with the Asian psyllid vector (Al-Zadjali et al.,2008), and might soon become, after Iran, the second country harbouring the two worst diseases of citrus: HLB and witches’ broom disease of lime.
As of January 2009, the countries around the Mediterranean basin (HLB. 005), as well as Australia, New Zealand, and North- and South-Pacific islands (HLB. 006) are free of both HLB and HLB-psyllid vectors, except Hawaii and Maui islands, which carry D. citri.
Table (HLB. 006b) summarizes the major characteristics of African, Asian, and American HLB.
Importance. HLB is a destructive disease, probably the most serious disease of sweet orange, mandarin and grapefruit trees, witches’ broom disease of lime being as bad, if not worse, for small-fruited, acid lime (C. aurantifolia). Once a tree carries Asian HLB, it will soon decline and become un-economical. Therefore, the only control method is prevention, i.e. preventing trees from becoming infected. It is estimated that close to 100 million trees are affected, worldwide. In the northern and eastern regions of Thailand, 95% of trees were affected as of 1981. In the Philippines, from 1961 to 1970, the citrus acreage was reduced by 60%. In Java and Sumatra, 3 million trees were destroyed from 1960 to 1970, and Bali lost 3.6 million trees from 1984 to 1987. In the south-western oases of Saudi Arabia, HLB, being spread by D. citri, had killed, by 1983, most sweet orange and mandarin trees. South Africa, at a time when Trioza erytreae was not yet seriously controlled with systemic insecticides, has paid a heavy toll to HLB. In the northern part of the country, in the White River region, citrus has been wiped out; in the Tzaneen region, citrus has been replaced by avocado; the Hazy View area has lost 90% of its citrus; and other regions are riddled with HLB (H. Le Roux, CRI, Nelspruit, S.A.) Free of HLB for many years, the Cape region of South Africa became affected with HLB in the late 1990s. Reunion Island lost its entire citrus in the 1960s. In SSP (Brazil), where HLB was recognized in March 2004, close to 3 million HLB-affected sweet orange trees have been eradicated since mid-2004 in the frame of the HLB-control programme. In Florida, HLB was reported in 2005. By 2008, the situation has become so serious, that the very existence of citrus is endangered, as no intensive control measures of HLB have been advocated and applied, except for some notable private efforts.
HLB is especially destructive on sweet orange, mandarin, tangelo and grapefruit trees, irrespective of rootstocks, whether the trees are grafted or are seedling trees. The crop on affected trees is not only reduced considerably by continuous fruit drop, dieback and tree stunting, but also by the very poor quality of fruits that remain on the trees.
The South African citrus industry has paid a heavy toll to HLB. However, since the late 1980s, when efficient control of the African psyllid was achieved with systemic insecticides as applied to the trunks or the roots of the trees, the disease is much less aggressive than its Asian counterpart in Brazil, Florida or Cuba. As African HLB is heat sensitive, the disease is severe in cool, elevated areas, while in dry, hot areas, the disease is mild or absent.
General reviews on HLB and its vectors have appeared: Bové (2006), da Graça (1991), da Graça and Korsten (2004), Garnier and Bové (1993), Gottwald et al. (2007), Halbert and Manjunath (2004).
NAME OF DISEASE AND SYNONYMS
When first observed in South Africa in the late 1920s, the disease was referred to as “yellow branch” in the western Transvaal, and as “greening” in the eastern Transvaal. The term “greening” prevailed and became the most common name of the disease, worldwide. It refers to the poorly coloured fruits, which, at the stylar end, are still greenish at harvest time. In the Chaozhou district of southern China (HLB. 001), the disease was known under the name huang long bing, “bing” standing for disease, “long” for shoot (and not for “dragon” in the local Chinese dialect!), and “huang” for yellow, hence: “huang long bing” = yellow shoot disease (and not yellow dragon disease). Huang long or yellow shoot is an early, characteristic symptom of the disease and refers to the yellow colour of the new flush of growth on infected trees (HLB. 007, 008). K. H. Lin, in the early 1950s, was the first to demonstrate by graft inoculation the infectious nature of the disease, for which he used the name “huanglongbing” (Lin, 1956). For these reasons, the International Organization of Citrus Virologists (IOCV) proposed in 1995 at the 13th conference of the IOCV in Fuzhou (Fujian province, China) that the official name of the disease be huanglongbing (abbreviation: HLB), and this proposal was accepted. Today, HLB is widely used for the African, American, and Asian forms of the disease.
Other names of the disease are as follows: “likubin” in Taiwan; “blotchy mottle disease” in South Africa, a very good name referring to the most characteristic leaf symptom (McClean and Schwarz, 1970); in the Philippines, the names “leaf mottling” (Salibe & Cortez, 1968) and “leaf-mottle-yellows” (Martinez and Wallace, 1968) also refer to leaf mottle, but lack the important term “blotchy”; in India, “citrus decline” or “citrus dieback” are unspecific names, as disorders other than HLB are often involved in citrus trees affected by decline or dieback; “vein-phloem degeneration” in Indonesia, draws attention on an important histological symptom.
Abbreviation: HLB for Huang Long Bing.
SYMPTOMATOLOGY
General aspect of affected field tree
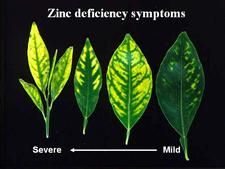
Sweet orange trees. In early stages of infection, affected trees are identified among symptomless trees by the presence of one (HLB. 009) or several characteristic “yellow shoots” (HLB. 010, 010b). With time, the yellow shoot grows into a larger yellow / pale green branch (HLB. 011). In South Africa, trees with large yellow branches are characteristic of HLB (HLB. 012) in comparison with symptomless trees (HLB. 013). In later stages of disease, the yellow branches take over the whole canopy, the tree is now fully (systemically) infected, and symptomatic all over (HLB. 014). In Sao Paulo state, the evolution of HLB from infected symptomless trees to fully affected trees (HLB. 015, 016, 017) is faster than in South Africa, and the “yellow branch” stage is less characteristic. Yellow branches may show one or several of the following features: defoliation, twig dieback, fruit drop, presence of leaves with blotchy mottle (see below), mineral deficiency patterns (zinc in particular), or uniformly yellow. Leaves with foliar symptoms of zinc deficiency have in general an upright growth, making a close angle with the shoot on which they are attached. Defoliation results in sparse foliage and an “open” type of growth (HLB. 018, 019). In Brazil, Florida and Cuba, when HLB was in the stages of spreading, many young orchards, because of abundant growth flushes, became affected more severely than old orchards with lesser flushes. While many trees in these orchards were fully affected (HLB. 019), other trees had still some symptomless sectors with well developed green leaves, while the symptomatic sectors had yellowish leaves with severe zinc deficiency patterns (HLB. 020, 021, 022, 023, 024). Such trees, with symptomless sectors and symptomatic sectors, are most characteristic of orchards invaded by HLB. In fall and early winter, these trees have blotchy mottle leaves, which may however drop in late winter and make HLB diagnosis less obvious.
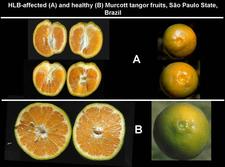
Mandarin and grapefruit trees. Nepal and Bhutan produce essentially local mandarins from seedling trees. Many orchards are severely affected by HLB as shown by photos (HLB. 025, 026, 027) for Nepal, and photos (HLB. 028, 029) for Bhutan. In Cuba, Dancy tangerine trees are also severely affected (HLB. 030, 031, 032). As the disease on mandarin trees progresses, more and more sectors turn yellow until no green leaves are left (HLB. 028, 029). Defoliation is severe (HLB. 027, 031, 032). In São Paulo State, HLB-affected Murcott tangor trees (HLB. 033) have pale green / yellowish branches, undergo defoliation and show severe fruit symptoms (see HLB. 098).
Photos (HLB. 034, 035, 036) show young grapefruit trees at various stages of affection in Cuba.
Symptoms on trunk (on rootstock and/or scion), limbs and shoots
No particular symptoms on trunk or limbs.
Symptoms on leaves
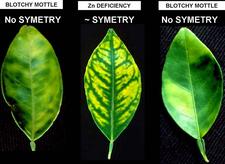
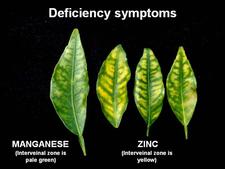
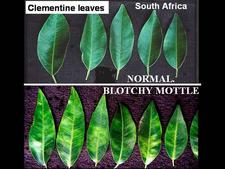
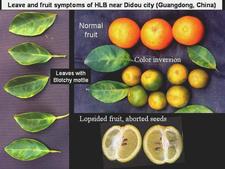
Symptomless sweet orange leaves are uniformly green (HLB. 037) One of the most characteristic symptoms of HLB, worldwide, is leaf “blotchy” mottle, to be distinguished from “simple” mottle. Photo (HLB. 038) shows Etrog citron leaves affected by “simple” mottle: the various shades of green do not blend into each other and are separated by sharp boundaries. The same is true for the variegated sour orange leaves of photo (HLB. 039). In contrast, the sweet orange leaf of photo (HLB. 040) shows “blotchy” mottle, characteristic of HLB. This leaf has several shades of yellow, pale green and dark green. These shades blend into each other, and there are no sharp boundaries between the various shades of colour, hence the term "blotchy" mottle. This symptom was described in particular by Lin (1956) in China (HLB. 041, 042), and McClean & Schwarz (1970) in South Africa (HLB. 043, 044, 044b), and it was seen again more recently in the state of São Paulo (HLB. 045, 046, 047), in Florida (HLB. 048, 049), and in Cuba (HLB. 050). In fact, blotchy mottle is synonymous with HLB and accompanies HLB wherever it occurs, be it in Nepal (HLB. 051), or Madagascar (HLB. 052). When examining the upper surface of most blotchy mottle leaves, the pattern on one side of the leaf-midrib is not symmetrical to that on the other side (HLB. O53). This asymmetry helps to distinguish blotchy mottle from deficiency symptoms due, in particular, to lack of zinc (HLB. 054, 055, 056), manganese (HLB. 057), or magnesium (HLB. 058). With trees in early stages of disease, blotchy mottle may affect large, well-developed leaves, and may be the only leaf pattern to be seen. In later stages, foliar symptoms of zinc deficiency will eventually develop, such leaves will remain small, and with time, the whole leaf blade may ultimately turn uniformly yellow (HLB. 059). Leaves may become thicker and leathery. Midribs and lateral veins are sometimes enlarged, swollen, and corky (HLB. 060, 061).
In certain situations, blotchy mottle is difficult to find. In 1996, in North Bali, Indonesia, HLB was severely affecting 4-year-old mandarin orchards. Most leaves were small and uniformly yellow. Some leaves had zinc deficiency symptoms, but clear-cut blotchy mottle was rare. PCR tests did however confirm HLB as the cause of decline of all the trees in these orchards. A similar situation was observed in Cuba in 2008.
Blotchy mottle is best observed on sweet orange leaves, but most other citrus species and varieties show it also, including grapefruit, pumelo, citron, and rough lemon (HLB. 062, 063), Citrus macrophylla (HLB. 064), Volkamer lemon (HLB. 065), sour orange (HLB. 066),…Leaves of lemon (HLB. 067, 068, 069, 070, 071), Mexican lime (HLB. 072, 073), and Tahiti lime (HLB. 074, 075) also show blotchy mottle, but of a type slightly different from that of sweet orange leaves (“lemon” type of blotchy mottle), as the patches of dark green tend to be much larger than in the case of sweet orange leaves. Some mandarin varieties such as Tejakula in Bali, Clementine in South Africa (HLB. 076) or Dancy tangerine in Cuba (HLB. 077), as well as tangelos (HLB. 078) show typical blotchy mottle symptoms. However, other mandarin varieties such as the Coorg mandarin in southern India (HLB. 079), or local mandarins in Nepal (HLB. 080, 081) and Bhutan, as well as Murcott tangors in São Paulo State do not present good, classic blotchy mottle. The areas of yellow, pale green and dark green are not as large as they are on sweet orange leaves, and are more like little “colour touches” rather than big “colour patches” (“mandarin” type of blotchy mottle). Also, affected mandarin leaves in the orchard tend to be more curled and twisted than sweet orange leaves (HLB. 079, 080). Often, HLB-affected mandarin trees have leaves with zinc deficiency symptoms in addition to leaves with “mandarin” type blotchy mottle (HLB. 082, 083). Sometimes, zinc deficiency patterns might be the only visible leaf symptoms.
Symptoms on fruit
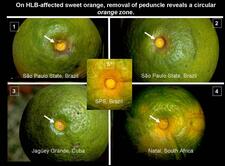
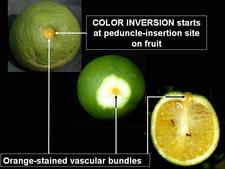
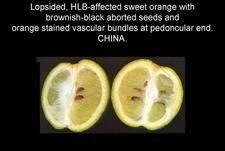
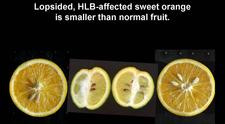
Sweet orange. In addition to blotchy mottle on leaves, HLB has also characteristic symptoms on fruit, easily seen on sweet oranges. On normal fruit, when fruit ripens and changes colour, the orange colour develops first at the stylar end, at a time when the peduncular end is still green (HLB. 084, 085). On HLB-affected fruit, there is colour inversion: the orange colour starts first at the peduncular end, at a time when the stylar end is still green (HLB. 084, 085). The “greening” aspect of HLB-affected fruit in South Africa is often due to colour inversion (HLB. 086), and when one presses such fruit with the thumb, a silvery greyish “finger-mark” appears on the rind surface (HLB. 087). Removing the peduncle from a totally green fruit reveals a depressed, circular zone, which is stained bright orange on HLB-affected fruit (HLB. 0881, 2, 3), and pale green on normal fruit (HLB. 089). Such an orange-stained peduncular scar is the first indication of color inversion; from there, coloration extends to the peduncular half of the fruit, down to the stylar end (HLB. 088 4, 5). On slide (HLB. 090), the fruit in the middle has been cut perpendicularly to the fruit axis, a few millimetres below the circular orange zone shown on the upper fruit; this section reveals the orange stained zone of the vascular bundles in the albedo. These vascular bundles are also visualized on the cross-section of the fruit in the lower part of the slide. Such cross-sections through the fruit axis are particularly useful to observe not only (i) the orange-stained vascular bundles in the peduncular half of the fruit axis (HLB. 090, 091, 093), but also (ii) the presence of aborted seeds .(HLB. 090, 091, 093), and (iii) the asymmetry or lopsidedness of the fruit (HLB. 091). Lopsided fruits are characteristic of HLB, and their size is smaller than that of normal fruits (HLB. 092). Small fruits with aborted seeds are also highly characteristic of HLB (HLB. 092). On HLB-affected fruit, the peduncle insertion point is often depressed because of the thickening of the albedo in the peduncular region (HLB. 093). Finally, HLB-affected trees are characterized by heavy fruit drop (HLB. 094, 095).
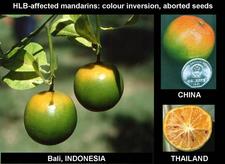
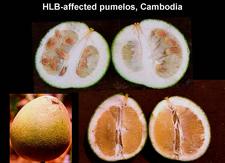
Mandarin, tangor, others. Colour inversion, small lopsided fruits, aborted seeds, and / or orange-stained vascular bundles are also observed on mandarins (HLB. 096, 097), tangors (HLB. 098), pumelos (HLB. 099), and many other species, hybrids or varieties.
Histological and cytological symptoms
Localized pockets of necrotic phloem are scattered through the leaves.
AGENTS ASSOCIATED WITH HLB: DESCRIPTION and PROPERTIES
1. CANDIDATUS LIBERIBACTER SPECIES.
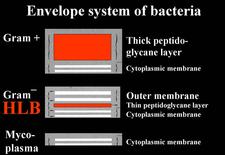
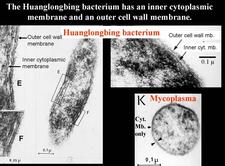
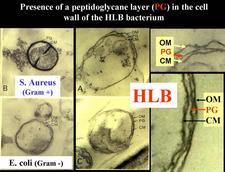
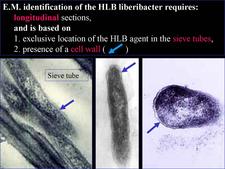
The agent associated with HLB is a sieve tube restricted bacterium, seen by electron microscopy (EM) for the first time in 1970 (Laflèche and Bové, 1970a) (HLB 100). Initially, the agent was thought to be a bacterium lacking a cell wall, i.e. a mycoplasma (Laflèche and Bové, 1970a, b). However, Bové and his group questioned the mycoplasmal nature of the HLB bacterium as early as 1971 (Saglio et. al., 1971). In South Africa, Moll et al. (1974) drew attention on the similarity between the cell-wall of the HLB agent and Gram negative bacteria. The Gram negative nature of the HLB bacterium was finally established in 1984 when it could be demonstrated by EM that the HLB agent possessed a cell wall of the Gram negative type (HLB. 101), composed of an outer membrane (HLB. 102) and an inner peptidoglycane layer (HLB. 1O3) (Garnier et al., 1984a, b).
In spite of many attempts, the HLB-associated bacterium could never be obtained in axenic culture, and thus Koch’s postulates could never be fulfilled. Therefore, it is improper to refer to the HLB-associated bacterium as the “causal agent”, the “agent” or the “pathogen” of HLB, and terms such as “HLB-associated bacterium” or “HLB-associated liberibacter” are to be used. The terms “HLB-organism”, “HLB-bacterium”, and “HLB-liberibacter”, as used occasionally here, are only abbreviations of the more proper terms involving “HLB-associated”. Recently, co-cultivation of the Asian HLB bacterium (Candidatus Liberibacter asiaticus) with actinobacteria has been reported (Davis et al., 2008). Also, a report on cultivation of Ca. L. asiaticus and Ca. L. americanus (Sechler et al., 2009) is in press while this chapter on HLB is being revised (January 2009).
Axenic cultures of the HLB-bacterium being unavailable in the 1990s, molecular techniques had to be used to characterize the organism at the phylogenetic and taxonomic level. The 16S ribosomal DNA (16S rDNA) of the HLB-bacterium could be obtained by PCR amplification and sequenced. Comparisons with known bacterial 16S rDNA sequences from data bases showed the HLB-organism to be indeed a Gram negative bacterium, as demonstrated earlier by EM, and more precisely a member of a new subgroup in the alpha subdivision of the Proteobacteria. Organisms in this subdivision live in intimate association with eukaryotic cells and, in many cases, have acquired the ability to survive and grow within an arthropod vector. The HLB-bacterium fits this description remarkably well. Indeed, it grows in a specialized niche in its eukaryotic plant host, the phloem sieve tubes, and it is transmitted by two arthropod vectors, the citrus psyllids Trioza erytreae and Diaphorina citri, in which it circulates and replicates. As the HLB-bacterium was the first representative of a new subgroup in the alpha-Proteobacteria, it was named “Liberobacter” from the Latin liber = bark and bacter = bacterium (Jagoueix et al., 1994). Liberobacter was later replaced by liberibacter (Garnier et al., 2000).
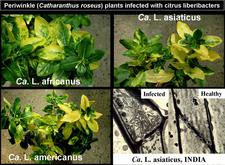
As indicated above, the HLB agent in Africa can be distinguished from the agent in Asia on the basis of temperature sensitivity (Bové et al., 1974), as well as DNA hybridizations and genomic properties (Villechanoux et al., 1992, 1993), and serology (Garnier et al., 1991; Gao et al., 1993). For these reasons, the two agents represent different bacterial species, and the agent in Africa has been denoted Candidatus Liberibacter africanus, while the agent in Asia is denoted Candidatus Liberibacter asiaticus (Jagoueix et al., 1994; Garnier et al., 2000b). The term “Candidatus” in front of a Latin binomial name indicates that the corresponding bacterium is not available in axenic culture and has been characterized by molecular, DNA-based techniques (Murray & Schleifer, 1994). Sequence studies of the region between the 16S rRNA gene and the 23S rRNA gene (16S/23S intergenic region) of African and Asian strains of the liberibacters have confirmed the notion that the African liberibacter and the Asian liberibacter represent two different liberibacter species (Jagoueix et al., 1997; Subandiyah et al., 2000).
A third liberibacter species has been identified in São Paulo state (SPS) when HLB was recognized in 2004 (see above: DISTRIBUTION). The sequences of the 16S rDNA and the 16S/23S intergenic region of the American HLB-bacterium have been compared to similar sequences of various isolates of the African and Asian liberibacters. These comparisons have shown that the American bacterium is a member of the genus Liberibacter, and represents a new species in this genus: Candidatus Liberibacter americanus (Teixeira et al., 2005a, b, c; 2008a). Additional properties of the American liberibacter fit those of the other two liberibacters. Transmission by graft-inoculation to healthy sweet orange seedlings has been obtained (HLB. 116), and EM observations showed the American liberibacter to be restricted to the sieve tubes (HLB. 104). When HLB was identified in 2004 in SPS, the American liberibacter was the major HLB agent, infecting more than 95% of the HLB-affected trees. The remaining trees were infected with Ca. L. asiaticus. Some trees carried both Ca. L. asiaticus and Ca. L. americanus. Since 2006, a decrease of Ca. L. americanus and an increase of Ca. L. asiaticus have been witnessed, in particular in the hotter northern regions of SPS, possibly because the American liberibacter (like the African liberibacter) is heat sensitive, while the Asian liberibacter is heat tolerant (Lopes et al, 2009b). Up to now, the American liberibacter was restricted to São Paulo and Minas Gerais states in Brazil. Recently, 330 symptomatic and symptomless leaf samples were collected from HLB orchards in Guangdong, Guangxi, Fujian, Jiangxi, Zhejiang, Hunan, Yuannan and Guizhou provinces of China. While Ca. L. asiaticus was detected in 96 samples, Ca. L. americanus was found in only one sample, a sample from Hunan (Lou et al., 2008). If this result is confirmed, it will be the first time that Ca. L. americanus has been detected out of Brazil. So far, the American liberibacter as well as the Asian liberibacter have never been detected in Africa.
Finally, a new liberibacter species, Candidatus Liberibacter solanacearum (Liefting et al., 2009a and b), has been found associated with diseases of solanaceous plants, tomato (Solanum lycopersicum), capsicum (Capsicum annuum), chilli (capsicum sp.), potato (Solanum tuberosum), tamarillo (Solanum betaceum), and cape gooseberry (Physalis peruviana) in New Zealand (Liefting et al., 2009a), and with Zebra chip disease of potato in the USA (Hong Lin et al., 2009). Zebra chip disease was first documented in potato fields in Mexico in 1994, and was first identified in the USA in 2000 in Texas (Gudmestad and Secor, 2007). The liberibacter is vectored by the potato / tomato psyllid, Bactericera cockerelli, from which the liberibacter has been characterized as Candidatus Liberibacter psyllaurous (Hansen et al., 2008). The Candidatus Liberibacter srtrain from the psyllid has also been characterized by Hong Lin et al. (2009). The psyllid has been recorded in the USA, Canada, Mexico, Guatemala and Honduras. Texas is the only location where the distribution of Diaphorina citri, the Asian citrus psyllid, and B. cockerelli overlap (Lia Liefting, personal communication). Citrus is not a host of B. cockerelli. The potato liberibacter is the first liberibacter to have been described from non-citrus hosts. It shares 95 to 97% 16SrDNA sequence identity with the citrus liberibacters and clusters, on the phylogeny tree, between the Candidatus Liberibacter africanus / asiaticus group and Candidatus Liberibacter amercanus. It is more closely related to Ca. L. africanus / Ca. L. asiaticus than to Ca. L. americanus. The latter diverged from other Ca. liberibacter spp. prior to divergence of other Ca. Liberibacter. spp. (Hong Lin et al., 2009).
In 1992, a Candidatus Liberibacter asiaticus strain from the Philippines was dodder-transmitted to tobacco (Garnier and Bové, 1993) (see below: HOST RANGE, non-rutaceous hosts) (HLB. 108, 109). Recently, a Florida strain of Ca. L. asiaticus was dodder-transmitted to tomato (Duan et al., 2008a).These results show that solanaceous plants are hosts of a citrus liberibacter. Inversely, the potato liberibacter can most probably be dodder-transmitted to sweet orange, and, if so, might even be associated with HLB. Once in citrus, it might be transmitted by the citrus psyllid, Diaphorina citri, from citrus to citrus…
Even though the liberibacters have not (yet) been available in culture, sequencing and annotation of about 90% of the genome of Ca. L. asiaticus could be achieved by using the technologies of multiple displacement amplification and 454 pyrosequencing. The draft genome of the liberibacter contained at least 1,161,087 base pairs with an average of 36% GC content. The genome contained three ribosomal operons, 34 tRNAs, and 1077 open reading frames of which 814 had a functional assignement (Douan et al., 2008b). Wulff et al. (2009) determined the size of the Ca. L. americanus genome by pulse field gel electrophoresis, using Lam-infected periwinkle plants for bacterial enrichment. The genome size was found to be close to 1.31Mbp. The data also suggest the genome to be circular and to contain three ribosomal operons.
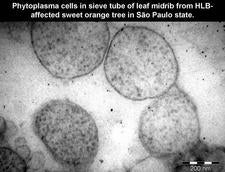
2. CANDIDATUS PHYTOPLASMA SPECIES
In early 2007, trees with characteristic leaf and fruit symptoms of HLB were discovered in two municipalities of northern SPS, hitherto free of HLB (HLB 105, HLB. 105b, 106). Surprisingly, these trees were PCR negative for all three liberibacter species. Eventually, the trees could be shown to be infected with a sieve tube restricted phytoplasma having 99% 16SrDNA sequence identity with the pigeon pea witches’ broom phytoplasma (group 16SrIX) (Teixeira et al., 2008c). Crotalaria junceae plants, grown in between citrus rows for soil improvement and showing characteristic witches’ brooms, (HLB. 106b) have been found infected with the HLB phytoplasma (Wulff et al., unpublished). Thus, a non-citrus host is probably the source of phytoplasma inoculum on which insect vectors such as leafhoppers, planthoppers or psyllids may become infected and transmit the phytoplasma to citrus. A phytoplasma has also been found associated with HLB in Southern China (Chen et al., 2008), but belongs to group16SrI, while the SPS phytoplasma is part of group 16SrIX.
Phytoplasmas (Plant mycoplasmas) are wall-less bacteria (Mollicutes) (HLB. 101, 127), and can be easily distinguished from the walled liberibacters (HLB. 101, 102, 103, 126) by electron microscopy.
HOST RANGE
Non-rutaceous hosts.
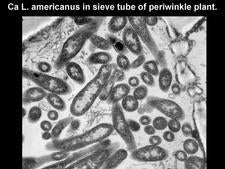
All three citrus liberibacters could be transmitted to periwinkle (Catharanthus roseus) plants by dodder (Cuscuta campestris): the Asian and African liberibacters in the early 1980s (Garnier and Bové, 1983), and the American liberibacter in 2005 (Bové J.M. and Bonnet P., unpublished). Symptoms in periwinkle plants are severe (HLB. 107). Once a HLB-infected periwinkle plant has been obtained by dodder transmission, it can be used as a source of infected shoots to graft-inoculate many additional periwinkle plants. The liberibacter titers in periwinkle plants are higher than in citrus. For instance, Ca. L. americanus reaches titers of 3.4 x 108 liberibacters per gram of midribs (Teixeira et al., 2008b).
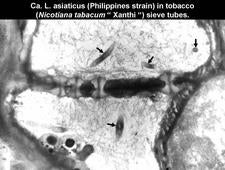
A Philippines strain of the Asian liberibacter was also transmitted to tobacco (Nicotiana tabacum Xanthi) by dodder (Garnier and Bové, 1993). Symptoms in tobacco (HLB. 108) were severe, even though the liberibacter titers were low (HLB. 109). Recently, Ca. L. asiaticus was dodder-transmitted to tomato (Duan et al., 2008a). These results show that a citrus liberibacter, can infect tobacco and tomato. It will be interesting to see if the liberibacter of solanaceous plants is able to infect citrus.
Sensitive species, varieties or combinations (Citrus and Citrus relatives). Nearly all seedlings and scion-rootstock combinations of citrus are sensitive to HLB, regardless of rootstocks and whatever the nature of the rootstock used.
Most severe symptoms are found on sweet orange, mandarin, tangelo, and grapefruit, followed by lemon, rough lemon, and sour orange. Severe fruit and leaf symptoms can be seen on Pumelo (Citrus grandis), even though this species is sometimes considered erroneously more or less tolerant. Trees of small-fruited, acid lime (Citrus aurantifolia) are only slightly affected, but clear-cut blotchy mottle symptoms can be seen on leaves. In many countries where HLB occurs, citrus tristeza virus (CTV) is also present and affects acid lime much more severely than does HLB. However, the lime trees are severely affected by the Asian HLB vector, D. citri, a real pest for lime trees (HLB. 115).
The ornamental citrus relative, Murraya paniculata (jasmin orange) (HLB. 110), shows leaf yellowing, defoliation and dieback on branches infected with the Asian or the American liberibacter in São Paulo state (Lopes et al., 2005 and 2006). M. paniculata was also found in Florida to be naturally infected with the Asian liberibacter (Zhou and Gabriel, 2007). Leaves of Clausena lansium (Chinese wampee) infected with the Asian liberibacter show leaf mottle (Ding et al., 2006). Severinia buxifolia (Chinese box orange) is also a host of the Asian liberibacter (Hung et al., 2000).
In the Western Cape province of South Africa, Calodendron capense, an ornamental rutaceous tree (Cape chestnut tree) (HLB. 110b), was found to be infected with a liberibacter. The new liberibacter was characterized as subspecies "capensis" of Ca. L. africanus (Garnier et al., 2000). Leaves from infected trees showed blotchy mottle (HLB. 110b). Recently, subspecies "capensis" was PCR-detected in several Calodendron trees without leaf symptoms (Phahladira, M.N.B., 2008).
Symptomless species, varieties or combinations
· Immune: none described
· Tolerant: trifoliate orange (Poncirus trifoliata), is said to be tolerant, but citranges may show leaf symptoms.
TRANSMISSION
Natural
- · Cultural transmission: through budwood.
- · Vector transmission (see also: distribution):
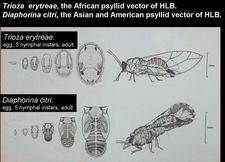
- by the African psyllid, Trioza erytreae (HLB. 111, 112)(McClean and Oberholzer, 1965b), in Africa, Madagascar island, Yemen,…
- by the Asian psyllid, Diaphorina citri (HLB. 111, 114) (Capoor et al., 1967; Martinez and Wallace, 1967) in Asia, South-East Asia, North-, Central-, and South-America where the Asian psyllid transmits both the Asian and the American liberibacter (Yamamoto et al., 2006), western Saudi Arabia, south and south-eastern Iran;
- by T. erytreae and D. citri in Reunion and Mauritius islands, and border region between Yemen and south-western Saudi Arabia.
- · Vector present but disease (still) absent (see also “Distribution”):
- T. erytreae present in Saint Helena island, Madeira island, and four Canary islands (Tenerife, La Gomera, La Palma and El Hierro).
- D. citri present in Argentina, Belize, Honduras, Venezuela, Costa Rica, Guadeloupe (France), Bahamas, Cayman Islands, Virgin Islands, Puerto Rico, Mexico, USA (Hawaii and Maui islands; all southern States of continental U.S., including Texas and California, and knowing that HLB is present in Florida and Louisiana), and Oman.
The psyllids can feed on many citrus species, but preferred hosts of D. citri are Murraya. paniculata (Orange jasmin, mock orange) (HLB. 110), M. exotica (Chinese box),and C. aurantifolia (HLB. 115), followed by Bergera koenigii (Curry leaf), and Clausena spp. Lemon (Citrus limon) is a good host of T. erytreae (HLB. 113). Psyllids need young, actively-growing foliage (flush) for development, and their populations peak at flush periods
Eggs of T. erytreae are seen mostly along edges of young leaves (HLB. 113). Those of D. citri occur on tips of growing shoots, on and between unfurling leaves. T. erytreae nymphs develop on the lower side of leaf blades (HLB. 113), while D. citri nymphs are found on young stems and petioles (HLB. 115). One major difference between the two psyllids concerns the effect of nymphs on leaf blades. T. erytreae nymphs are embedded in pits or nests on the lower side of the leaves (HLB. 113 A); these pits look like hemispherical bumps on the upper side (HLB 113 B, C). After an adult has emerged from a nymph, the nest is empty, but the bump remains. Hence, the presence of even one single bump on one single leaf of one single tree is proof of T. erytreae occurrence. No such bumps are produced by D. citri., as the nymphs do not develop on leaves, but on young, little stems and petioles (HLB. 115). Leaves with bumps may be severely distorted. With D. citri, leaves are heavily curled and may be covered with honeydew (HLB. 115). Characteristically, D. citri adults form a 45° angle with the plant tissue on which they feed (HLB. 114). T. erytreae multiplies and produces bumps on all citrus species and varieties, as well as on non-citrus rutaceous hosts such as Vepris undulata, Clausena anisata, Zanthoxylum (Fagara) capense, Toddalia lanceolata (HLB. 113 C).
The putative insect vector of the HLB phytoplasma in SPS has not yet been identified.
- · Is there seed transmission of the HLB-liberibacters?
Seed transmission of Ca. L. asiaticus has been reported (Zhou et al., 2008a), It is is based on PCR detection of the liberibacters in some seeds (~2%) from HLB-affected citrus fruits, as well as in the progeny seedlings grown from these seeds. However, the detected liberibacters were present in very low titers and required improved techniques such as nested PCR for their detection. In addition, the citrus progeny seedlings did not show blotchy mottle. They did show some atypical symptoms when they were stressed by nutritional deficiency, but these symptoms disappeared when the stress was removed (Zhou et al., 2008b). Whatever the reason of the very mild PCR reactions observed, genuine seed transmission implies that the disease, HLB, shows up in the progeny seedlings, in the same way that the disease shows up in graft-inoculated seedlings (HLB. 116). Also, the titer of liberibacters in the symptomatic progeny seedlings should be similar to that in the symptomatic graft-inoculated seedlings. For these reasons, it seems that seed transmission of HLB has not yet been demonstrated.
Experimental
Mechanical transmission: unsuccessful.
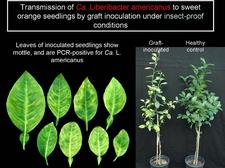
Graft inoculation with shoots, buds, and bark from shoots or roots, leaf patches (HLB. 116).
Transmission of Ca. L. americanus to various citrus species and cultivars by graft-inoculation has been studied recently in the greenhouse (Lopes et al., 2008). Leaf mottling and symptoms of mineral deficiencies appeared 4 to 5 months after inoculation. Blotchy mottled leaves were only seen on sweet orange and Murcott tangor plants, and transmission percentages were higher for these cultivars (31.2 to 65.2 %) than for limes, mandarins, and Swingle citrumelo (2.0 to 25 %). It was also found that the larger the pieces of tissue used for inoculum, the higher the percentages of transmission. Small, lopsided fruits with brownish columellae developed on sweet orange plants.
The titer of Ca. L. americanus in field trees (5.74 log cells/g leaf midrib) was found to be lower than that of Ca. L. asiaticus (6.67 log cells/g leaf midrib), and the transmission percentages by graft inoculations were also lower for the former (10.0 to 45.2%) than for the latter (54.7 to 88.0 %) (Lopes et al, 2009a).
Transmission by dodder (Cuscuta campestris) from citrus to periwinkle, tobacco and tomato. See above: non-rutaceous hosts.
Adults and nymphs of D. citri take 30 minutes to acquire the Asian liberibacter, while 24 hours are needed for T. erytreae to acquire the African liberibacter. Adults and fourth and fifth nymphal instars of D. citri can transmit the bacteria after a latent period as short as one day or as long as 25 days (Xu et al., 1988). Capoor et al. (1974) observed that the latent period of the Asian liberibacter in adult Asian psyllids was 8 -12 days. For the African psyllid, a latent period of 24 hours was reported (Buitendag and von Broembsen, 1993). The inoculation time for the Asian pysllid to transmit the Asian Liberibacter is more than 1.5 hours. Transmission parameters have not yet been determined for Candidatus Liberibacter americanus, which is also transmitted by D. citri.
EPIDEMIOLOGY (by Renato Bassanezi)
HLB epidemics take several years to reach high incidence levels. The temporal progress of HLB incidence is dependent on (i) vector populations, (ii) extent of the inoculum reservoir, and (iii) age of the grove at first infection. The disease progression in the orchard can be relatively fast, reaching more than 0.95 incidence in 3 to 13 years after onset of the first symptoms (Aubert et al, 1984; Catling & Atkinson, 1974; Gatinau et al., 2006; Gottwald et al., 1989; Gottwald et al., 1991; Gottwald et al., 2007, Gottwald et al., 2009). In young groves (up to 3 years old), 3 to 5 years have been necessary for disease incidence to reach the asymptotic levels, while in older groves more than 5 years were needed. The evolution of symptom severity can be very fast until the symptoms are distributed throughout the tree canopy and become very severe. Severe symptoms have been observed 1 to 5 years after onset of the first symptoms, depending also on the age of the tree at infection time, but also on the number of multiple infections (Aubert, 1992; Lin, 1963; Schwarz et al., 1973). As the disease severity increases, the yield is reduced and makes the orchard economically unfeasible in 7 to 10 years after planting (Aubert, 1990; Aubert et al., 1984; Gottwald et al., 1991; Roistacher, 1996).
Gottwald et al (2007) have recently reviewed the literature on HLB epidemiology and pointed out the difficulties of conducting quantitative epidemiological studies on HLB. Due to the devastating effects of HLB and the fear of allowing uncontrolled inoculum sources to exist in a region with susceptible plantings, it has been difficult to locate study sites where the disease is allowed to progress unimpeded, so that epidemics can be followed over multiple years without intervention of control activities. In addition, monitoring the occurrence of symptoms can be somewhat problematic because of the variable time lags between transmission of the pathogen by psyllid vectors and the onset of visual symptoms. Natural transmission due to insect vectors is probably greatest when new flush is available, increasing psyllid populations and migrations. Symptom expression usually occurs during autumn and winter (HLB. 117). Thus, trees infected at different times may express onset of symptoms at the same time. At a given time, due to the delay between infection by the vector and symptom expression, some infected trees are already symptomatic, while others are still symptomless. It has been found that the number of infected but symptomless trees equals roughly the number of symptomatic trees (Irey et al., 2006), so that when a given visual inspection reveals "n" symptomatic trees, it can be assumed that the total number of infected trees is in fact ~2n.
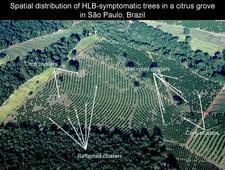
The spatial dynamics of HLB have been investigated primarily in Reunion Island and China (Gottwald et al., 1989; Gottwald et al., 1991). More recently, studies have been conducted in Sao Paulo State (Bassanezi et al., 2005) and Florida (Gottwald et al., 2009). Usually, higher concentrations of symptomatic trees are initially found at the borders of blocks (edge effects) (HLB. 118). Some evidence of clustering among immediately adjacent diseased trees was demonstrated, but was not particularly strong. In many cases, within-row aggregation was slightly stronger than across-row aggregation. Aggregation of diseased trees within groups was demonstrated for all plots at all locations and all quadrat sizes except when disease incidence was extremely low or high. Core clusters of HLB-infected trees were found to be associated with secondary clusters (reflected clusters) as far as 25 to 50 m apart (HLB 119). Thus, in a local scale, vector movement appears to be generally from one tree to the next as well as to trees at 25 to 50 m distance, initiating new foci of infection. Also, there are strong indications of regional spread of HLB. Continuous relationship among HLB-diseased trees over a broad range of spatial distances up to 3.5 km was observed. An estimate of the most common distance between pairs of HLB-infected trees ranged from 0.88 to 1.61 km with a median of 1.58 km, which may indicate an average psyllid dispersal distance from a regional point of view (Gottwald et al., 2009). In conclusion, HLB seems to spread through an incessant mixture and alternation of (i) random primary infections (‘’background’’ transmission) resulting from infective psyllids that periodically emigrate from HLB sources outside the plots and (ii) secondary infections (‘‘local’’ transmission) that operate over short distances with infective psyllids within the plot.
DIAGNOSIS
Diagnostic field symptoms
When HLB enters a region hitherto free of the disease, young trees show symptoms earlier, are more severely affected, and have a more rapid disease evolution than adult trees (HLB. 019, 032, 036). Young trees may be fully infected or still have some symptomless sector(s) (HLB. 020, 022).
In general, trees are not uniformly affected. According to the time of infection, the affected sector may be a single yellow shoot or a whole yellow branch. These yellow shoots or branches, frequently with sparse foliage and “open” type growth, stand out against the green canopy of the tree (HLB. 010b, 012). Eventually, the whole tree is affected (HLB. 014, 017). Affected parts have often leaves showing blotchy mottle symptoms (HLB. 042), one of the most characteristic diagnostic features of HLB. Leaves with zinc deficiency symptoms may also be seen (HLB. 059), or even be the only symptomatic leaves present, when blotchy mottle leaves have dropped or when blotchy mottle is not well expressed in the spring/summer months.
Fruits have also highly characteristic symptoms. They are small, lopsided, and show often colour inversion (HLB. 085, 096). When such fruits are cut in half along the fruit axis, aborted, brownish-black seeds are frequently seen (HLB. 091), and the vascular bundles at the pedoncular end of the fruit axis are stained orange-brown (HLB. 090). Slide (HLB. 120) summarizes the leaf and fruit symptoms. Comparison with other diseases.
The zinc deficiency symptoms associated with HLB can be confused with genuine zinc deficiency (HLB. 055). HLB zinc deficiency is usually sectorial and is found on some trees (those affected by HLB) but not on others. Genuine zinc deficiency is found on a large number of trees in a regular distribution pattern within an orchard. Zinc deficiency symptoms can be corrected by zinc sprays in both HLB-affected trees and trees with genuine zinc deficiency symptoms, but the fruit symptoms of HLB are not corrected by such sprays.
Until 1970, when stubborn disease and HLB were not yet recognized as different bacterial diseases, it was generally assumed that they were “strains” of the same “virus” because they had some symptoms in common. Stubborn trees may show leaf symptoms that resemble mild blotchy mottle. Even though the most characteristic stubborn fruits are those with an “acorn” shape (HLB. 121), lopsided fruits with colour inversion and aborted seeds can also be found on stubborn trees, and resemble those from HLB trees (HLB. 122). Differences between the two diseases concern the foliage. Stubborn trees have a dense, bushy aspect, with shoots having short internodes and carrying rosettes of rather small, spoon-shaped leaves. Affected sectors of HLB trees have sparse foliage and characteristic blotchy mottle leaves and/or leaves with zinc deficiency symptoms. Off-season flowering is more pronounced with stubborn disease than with HLB. Asian HLB and stubborn disease are both heat tolerant, but African HLB is heat sensitive. The agents associated with the two diseases, Spiroplasma citri for stubborn and Ca. Liberibacter spp. for HLB, belong to different bacterial divisions: the Mollicutes (mycoplasmas) in the case of S. citri, and the alpha-Proteobacteria (Gram negative bacteria) for Ca. Liberibacter spp. However, the two different bacterial agents have the same in situ location in the affected plants: the phloem sieve tubes. Both bacterial agents are transmitted by insect vectors feeding on phloem sap in the sieve tubes, but the insect vectors are psyllids for HLB, and leafhoppers for stubborn.
Like S. citri, phytoplasmas are also mollicutes. In SPS, a phytoplasma of group 16SrIX (Teixeira et al., 2008b) and, in southern China, a phytoplasma of group 16SrI (Chen et al., 2008) are associated with HLB symptoms. However, other phytoplasmas infecting citrus, such as the lime witches’ broom phytoplasma, Candidatus Phytoplasma aurantifolia (group 16SrII), are not associated with HLB symptoms. The upper side of leaves showing symptoms of Citrus Variegated Chlorosis (CVC) (HLB. 123 A) resemble zinc deficiency patterns (HLB. 054) and should not be confused with HLB-induced zinc deficiency symptoms. Characteristically, the lower sides of CVC-affected leaves, but not those of zinc-deficiency leaves, have a brownish stain at the positions of the yellow patches (HLB. 123 B), the centers of which might become necrotic.
Biological indexing
Preferred indicator seedlings are sweet orange (Madam Vinous, Pineapple, Hamlin,… (HLB. 116) and tangelo (Orlando, Seminole) varieties. Inoculation can be with budwood sticks, bark, buds, and patches of blotchy mottle leaves from affected parts of the candidate tree. Transmission is not 100% successful, probably because of low concentration and uneven distribution of the liberibacters, and 5 to 10 indicator seedlings should be used for each source to be indexed. After inoculation, the indicator seedlings, inoculated with the temperature-sensitive African liberibacter or American liberibacter, should be kept at cool temperature conditions (20°C for 8 hours in the dark, and 24 to 27°C for 16 hours in the light). For the temperature-tolerant Asian liberibacter, temperatures can be higher (25°C for 8 hours in the dark, and 30 to 32°C for 16 hours in the light). See also: TRANSMISSION, experimental.
Electron microscopy
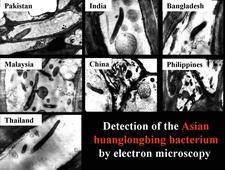
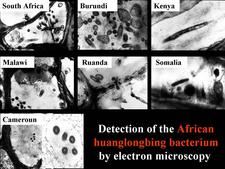
From 1970 to 1990, transmission electron microscopy (TEM) has been the first and only laboratory technique for indisputable identification and confirmation of HLB, and has been widely used (Garnier and Bové, 1996) (HLB. 124, 125). The reliability and specificity of TEM is based on two properties of the HLB liberibacter: its exclusive location in the sieve tubes, and the presence of a cell wall (HLB. 126). In citrus, no bacteria other than the HLB-associated liberibacters fit these properties. For TEM detection, midribs of blotchy mottle leaves are used. Longitudinal ultrathin sections are to be preferred over cross sections. TEM is a heavy and time-consuming technique, and cannot distinguish between African, Asian or American liberibacters, but can distinguish between the walled liberibacters (HLB. 126) and the wall-less HLB-phytoplasmas (HLB. 127).
Serological diagnostic methods
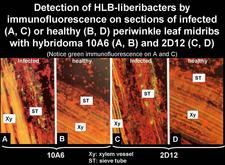
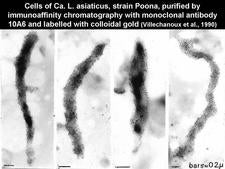
Thirteen monoclonal antibodies (MA) specific for the African or Asian liberibacters have been produced (Garnier et al., 1991; Gao et al., 1993). The use of these MAs for the detection of the HLB liberibacters by immunofluorescence on thin sections (HLB. 128) has shown that each MA is very specific for the strain used for immunisation and, therefore, it is not advisable to use the MAs, either singly or in cocktails, for generalized diagnosis of HLB (Garnier et al., 1991). They have however been used for purification of the liberibacter cells (HLB. 129).
Molecular diagnostic methods
Today, molecular techniques, DNA hybridization and in particular Polymerase Chain Reaction (PCR), are the techniques of choice for HLB diagnosis. The plant DNA required for these techniques is obtained from leaf midribs by the CTAB method of Murray and Thompson, 1980. If possible, leaves with blotchy mottle symptoms should be used, as the liberibacter titer in these leaves is generally high (Teixeira et al., 2008b).
DNA hybridization. The rplKAJL-rpoBC gene cluster codes for ribosomal proteins K, A, J, L, and RNA polymerase proteins B and C. The gene clusters from Ca. L. asiaticus and Ca. L. africanus were obtained and used, respectively, as hybridization probes In-2.6 for specific detection of the Asian liberibacter, and probe As-1.7 in the case of the African liberibacter (Villechanoux et al., 1992, 1993; Planet et al., 1995). These probes can also be used to detect the liberibacters in psyllid vectors. Individual insects are crushed on a nylon membrane, and the membrane or “crush-blot” is submitted to hybrydization with one or the other probe (Bové et al., 1993). Recently, a similar probe has been obtained from the equivalent gene cluster of Ca. L. americanus (Teixeira et al., 2008a).
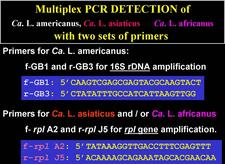
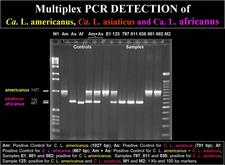
Convential PCR. Two PCR systems have been used. The first is based on the amplification of a 1160 bp fragment of liberibacter 16S rDNA with primer pair f-OA1/r-OI2c for Ca. L. africanus and f-OI1/r-OI2c for Ca. L. asiaticus (Jagoueix et al., 1996). With primers f-GB1/r-GB3 for Ca. L. americanus, a 1027 bp amplicon is obtained (Teixeira et al., 2005b). The 1160 amplicon from the Asian liberibacter yields two restriction fragments (520 bp and 640 bp) when treated with Xba1, and can thus be easily distinguished from the 1160 amplicon of the African liberibacter, which yields three fragments (520 bp, 506 bp and 130 bp). The second PCR system is based on the rplKAJL-rpoBC gene cluster (see above). With primer pair f-rplA2/r-rplJ5, a 701 bp amplicon is obtained with the Asian liberibacter, and a 667 bp amplicon with the African amplicon, because the intergenic region between genes A and J is 34 bp larger in tne Asian liberibacter as compared to the same region in the African liberibacter (Hocquellet et al.,1999)
A “multiplex” PCR system for routine detection of all three liberibacters in one test tube within a single reaction mixture containing the two sets of primers described above (f-GB1/r-GB3 for 16S rDNA amplification of Ca. L. americanus, and f-rplA2/r-rplJ5 for rpl gene amplification of Ca. L. africanus and Ca. L. asiaticus) has been developed (HLB. 130, 131).
Nested PCR. 16S rDNA of either one of the three liberibacters is first amplified with universal PCR primers f-FD1 and r-RP1 (Weisburg et al., 1991). The PCR product is diluted and reamplified with primers f-GB1/r-GB3, OA1/r-OI2C, or f-OI1:r-OI2c, whether Ca. L. americanus, Ca. L. africanus, or Ca. L. asiaticus is involved.
Real Time PCR. Recently, real time PCR (RTi-PCR) and quantitative real time PCR (q-PCR) have been applied to the detection and quantification of liberibacters in plants and insect vectors (Li et al., 2006 and 2007; Wang et al., 2006; Teixeira et al., 2008b). The sensitivity of RTi-PCR (SYBER Green) for detection of Ca. L. americanus in leaf samples from the field (Teixeira et al., 2008b) was such that a positive amplification was still obtained with only 10 liberibacter cells per PCR reaction mixture (HLB. 132). Nested PCR (n-PCR) was almost as sensitive as RTi-PCR (HLB. 132). Conventional 16SrDNA PCR (c-PCR) was 1000 times less sensitive (HLB. 132). Yet, in routine testing for liberibacter in leaf samples from the field, c-PCR gave positive reactions with practically all leaf samples showing blotchy mottle. Blotchy mottle leaves test positive by c-PCR because they have the highest titers of liberibacters, and this is true for all three liberibacter species. q-PCR has shown blotchy mottle leaves to contain an average value of 107 liberibacters per gram of leaf midribs. Even the lowest liberibacter titers in blotchy mottle leaves are 10 times higher than the limit of detection of c-PCR.
RTi-PCR has recently been shown capable of detecting the Asian liberibacter in symptomess trees of affected orchards (Irey et al., 2006), and the American liberibacter in symptomless leaves of affected trees (Teixeira et al., 2008b).
CONTROL
Antibiotic treatments.
Bacteria, but not viruses, are sensitive to antibiotics. Immediately after the discovery, in 1970, that HLB was associated with a bacterium, and not a virus, tetracycline injections into the trunks of HLB-affected citrus trees were tried in South Africa, and found to reduce significantly the incidence of symptomatic fruit (Schwarz & Van Vuuren, 1971; Schwarz et al., 1974; Moll & Van Vuuren, 1977; Moll et al., 1980). The control procedure was however stopped after a few years. Indeed, tetracycline is only bacteriostatic, not bacteriocidal, and treatments had to be repeated each year. After several trunk injections the antibiotic induced phytotoxicity in the injected citrus trees. Also, the treatment was not precisely environment friendly. Tetracycline injections were used for some time in Taiwan (Su and Chang, 1976; Chiu et al.,1979) and Indonesia (Supriyanto and Whittle, 1991) without appreciable results. Experimentally, penicillin was shown to give remission of HLB symptoms, and this result supported the bacterial nature of the HLB agent (Aubert and Bové, 1980; Bové et al., 1980).
Preventive control of HLB in São Paulo State, Brazil.
In the absence of curative treatments, preventive control, i.e. preventing citrus tree from becoming infected with the HLB-associated agent, is the only way to “cope” with HLB, once the disease has become established in a region. Preventive control is based on three sound measures: 1) elimination of liberibacter inoculum by removal of symptomatic trees, 2) insecticide treatments to keep psyllid vector populations as low as possible, and 3) production of healthy citrus trees in “closed”, insect-proof nurseries (HLB. 133) for new orchards as well as replacements of removed symptomatic trees.
Inspections for identification and removal of symptomatic trees. All trees in a grove, however big the grove, must be inspected one by one to identify the symptomatic trees, and at least four inspections must be carried out per year. Indeed, infected trees are of two types: those that, at a given inspection, show symptoms and those that show no symptoms yet. At survey # n, only trees showing symptoms can be identified and removed. Infected trees without symptoms can only be identified later, when they show symptoms. Also, between inspections, psyllids that have escaped control may infect additional trees. Finally, there are “escape” trees, i.e. symptomatic trees missed at a previous inspection. Hence, additional inspections must be conducted to “catch” all these trees. In the large groves of São Paulo state and Florida, inspections of adult trees are conducted with “platforms” on which two (HLB. 134, 135, 136, 138) or four (HLB. 137) inspectors take place. The platform moves in between rows at a speed of 3 to 4 km/hour, inspects ~5000 trees/day, and allows excellent inspection of tree tops. Even for young orchards, platforms with two inspectors are now used, and avoid inspections by ground inspectors (HLB. 134). Finally, symptomatic trees, which have been identified during the inspection, must be removed as quickly as possible, within a few days following identification (HLB. 139). After tree removal, sprouts may grow from the stump and/or roots that have remained in the soil (HLB. 139 D); these sprouts, stumps and roots must be carefully eliminated.
In South Africa, inoculum reduction was based only on cutting off affected branches rather than removing the whole tree. It has been shown in Brazil (Lopes et al., 2007), and now also in South Africa, that, generally, branch removal does not cure the tree and is not the best way of decreasing liberibacter inoculum.
Control of psyllids. Treatments with contact and systemic insecticides reduce psyllid populations, and thus HLB spread. Key features of the psyllids, which need to be taken into account in the formulation of control strategies, have been reviewed (Buitendag & von Broembsen, 1993; Halbert & Manjunath, 2004, Manjunath et al., 2008).
In South Africa, systemic insecticides, aimed specifically at psyllids (monocrotophos) have been applied directly to the tree trunks (HLB. 140), and have been very effective. In Asia, a large range of insecticides, mostly organophosphates and pyretroids, are used in very intensive spray programs to kill eggs and nymphs on flush growth. Horticultural and agricultural mineral oils are being developed as alternative treatments, much less damaging to the environment and less disruptive to biocontrol of other pests (Davis et al., 2005a).
In São Paulo state, according to the severity of the HLB infection, preventive control of HLB involves aggressive psyllid control implying 6 to 24 annual insecticide applications with ground sprayers, and 4 to 8 annual applications by airplane, associated with 4 to 12 annual platform inspections for identification and removal of symptomatic trees. Similar control programs are envisaged in Florida. These programs are meant to eventually keep the percentage of symptomatic trees well below the 1% level at each inspection. Finally, removal of symptomatic trees without psyllid control, or psyllid control without symptomatic tree removal will only lead to ineffective HLB control.
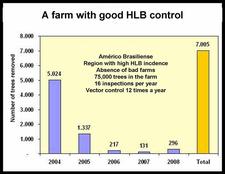
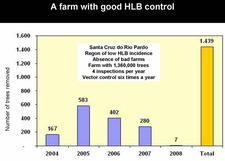
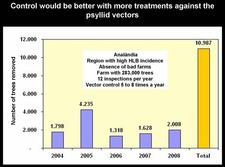
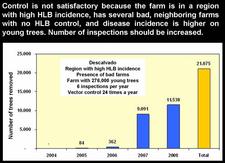
São Paul state started to control HLB very soon after the disease was recognized in March of 2004. After four years of HLB control in SPS, several critical points have been identified (Belasque et al., 2008). Large citrus farms, with old trees, in a region with low HLB incidence, far away from “bad” farms (i.e. farms without HLB control), with control having started as early as HLB appeared and involving 12 rather than 4 annual inspections for symptomatic trees, and 24 rather than 6 annual insecticide treatments, achieve a higher quality of control than farms with opposite characteristics (see table). Two citrus farms with good HLB control are illustrated on slides (HLB. 141) and (HLB. 142). In these farms, the number of symptomatic trees removed in 2008 was very much smaller than the number of symptomatic trees removed in 2004 and / or 2005. Farm of slide (HLB. 143) is located in the region of high HLB incidence, but has no “bad” neighbouring farms; the farm could probably have achieved a better control with a higher number of insecticide treatments. In farm of slide (HLB. 144), HLB is not under control. The trees were planted in 2004 and are still very young. They are under irrigation and produce abundant flushes of growth. The major problem of this farm is the presence, in close vicinity, of several “bad” farms where no HLB control has ever been done. The contaminated psyllids from the “bad” farms are attracted by, and move to, the “good” farm, which they contaminate. An even more aggressive vector control, with systemic insecticides applied to the soil or the trunks, has to be carried out as long as the “bad” neighbouring farms have not been removed.
Biological control of psyllid vectors.
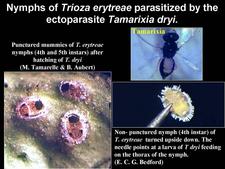
Reunion island is one of the very few regions where biological control of the two psyllid vectors of HLB has been effective (Etienne and Aubert, 1980; Aubert and Quilici, 1984). Diaphorina citri used to be present below 400-500m, and Trioza erytreae, above 400-500m (HLB. 145). High populations of these insects were observed at each flushing period. An endoparasite of D. citri, Psyllaephagus harrisoni, was present in the island, but turned out to be only slightly effective for control. Hence, it became necessary to introduce efficient parasites from abroad and increase them by rearing on the two psyllid hosts before releasing them in the field. Tamarixia (ex-Tetrastichus) dryi, an ectoparasite of T. erytreae (HLB. 146), was imported from Transvaal, South Africa, in December 1974. Tamarixia radiata, anectoparasite of D. citri, was imported in April 1978 from the Punjab, India. The fact that these psyllid parasites were initially absent from the island, made it sure that their own parasites were also absent. Therefore, great care was taken to avoid introduction of Tamarixia parasites when introducing the two Tamarixia species. Absence of Tamirixia parasites (or Psyllid hyperparasites) is one of the reasons, which explain the success of biological control of psyllids in Reunion island. A second reason is the fact that Tamarixia dryi was able to parasitize, and multiply on, not only Trioza erytreae, but also an additional psyllid, Trioza eastopi, living on a very common Reunion shrub, Litsea chinensis. In this way, important populations of T. dryi were obtained.
Protection against HLB by Guava plants
In the Mekong delta of Vietnam, farmers have found that the presence of guava trees close to citrus trees prevents or at least retards huanglongbing. It has recently been shown that the volatiles of guava repel D. citri, and this explains the guava-tree effect (Noronha et al., 2008). While planting one or several guava trees next to each citrus tree does not seem to be a very practical solution for large orchards, a repellent based on the guava volatiles or even a citrus plant genetically modified to produce the active volatile(s) might be an elegant solution to control HLB.
SELECTED REFERENCES
Al-Zadjali, T. S., Ibrahim, R., Al-Rawahi, A. K. (2008). First report of Diaphorina citri from the Sultanate of Oman. Insecta Mundi 0039: 1-3.
Asana, R.D. (1958). The citrus dieback problem in relation to cultivation of citrus fruits in India. Indian Journal of Horticulture 15: 283-286.
Aubert, B. (1990). Integrated activities for the control of huanglungbin-greening and its vector Diaphorina citri Kuwayama in Asia. p. 133-144. In B. Aubert, S. Tontyaporn and D. Buangsuwon (eds.) Rehabilitation of Citrus Industry in the Asia Pacific Region. Proc. Asia Pacific Intern. Conf. on Citriculture, Chiang Mai, Thailand , 4-10 February 1990. UNDP-FAO, Rome.
Aubert, B., Bové, J.M. (1980). Effect of penicillin or tetracycline injections of citrus trees affected by greening disease under field conditions in Reunion island. Proc. 8th Conf. IOCV, 103-108, IOCV,Univ. Calif., Riverside 1980,.
Aubert, B., Quilici, S. (1984). Biological control of the African and Asian citrus psyllids (Homoptera: Psylloidea) through Eulophid and Encyrtid parasites (Hymenoptera: Chalcidoidea) in Reunion island. Proc. 9th Conf. IOCV, 100-108, IOCV, Univ. Calif., Riverside, CA.
Aubert, B., Bové, J.M., Etienne, J. (1980). La lutte contre la maladie du « greening » des agrumes à l’île de la Réunion. Résultats et perspectives. Fruits 35: 605-624.
Aubert, B. (1992). Citrus greening disease, a serious limiting factor for citriculture in Asia and Africa. Proc. Intern. Soc. Citricult. 817-820.
Aubert, B., Sabine, A., Geslin, P., Picardi, L. (1984). Epidemiology of the greening disease in Reunion Island before and after the biological control of the African and Asian citrus psyllas. Proc. Intern. Soc. Citricult. 1: 440-442.
Augier, L., Gastaminza, G., Lizondo, M., Argañaraz, M., Willink, E. (2006). Presencia de Diaphorina citri (Hemiptera: Psyllidae) in North West Argentina. Rev. Soc. Entomol. Argent. 65: 67- 68.
Bassanezi, R.B., Busato, L.A., Bergamin Filho, A., Amorim, L., and Gottwald, T.R.
2005. Preliminary spatial pattern analysis of Huanglongbing in São Paulo, Brazil. Proc. 16th Conf. IOCV., 341-355, IOCV, Univ. Calif., Riverside, CA.
Belasque Jr., J., Bassanezi, R. B., Yamamoto, P. T., Lopes, S. A., Ayres, A. J., Barbosa, J. C., Tachibana, A., Violante, A. R., Tank Jr., A., Giorgetti, C. L., Di Giorgi, F., Tersi, F. E. A., Menezes, G. M., Dragone, J., Catapani, L. F., Jank Jr., R. H., Bové, J. M. (2008). Factors associated with control of huanglongbing in Sao Paulo, Brazil: a study of cases. Int. Research Conf. Huanglongbing, Dec. 1- 5, Orlando, Fl., USA.
Bonavia, E. (1888). The cultivated oranges and lemons of India and Ceylon. W.H. Allen & Co., London.
Bové, J. M. (2006). Huanglongbing: a destructive, newly-emerging, century-old disease of citrus. J. Plant Pathology 88: 7-37.
Bové, J.M., Calavan, E.C., Capoor, S.P., Cortez, R.E., Schwarz, R.E. (1974). Influence of température on symptoms of California stubborn, South African greening, Indian citrus decline and Philippines leaf mottling diseases. Proc. 6th Conf. IOCV, 12-15, Univ. Calif., Div. Agr. Sci., Riverside. CA.
Bové, J.M., Bonnet P., Garnier M., Aubert B. (1980). Penicillin and tetracycline treatments of greening disease-affected citrus plants in the glasshouse, and the bacterial nature of the procaryote associated with greening. Proc. 8th Conf. IOCV, 91-102, IOCV, Univ. Calif., Riverside, CA.
Bové, J.M., Erti Dwiastuti, M., Triviratno, A., Supriyanto, A., Nasli, E., Becu, P., Garnier, M. (2000). Incidence of huanglongbing and citrus rehabilitation in North Bali, Indonesia. Proc.14th Conf. IOCV, 200-6, IOCV, Univ. Calif., Riverside, CA.
Bové, J.M., Garnier, M. (1984). Citrus greening and psylla vectors of the disease in the Arabian Peninsula. In: Proc. 9th Conf. IOCV, 109-14, IOCV, Univ. Calif., Riverside, CA.
Bové, J.M., Garnier, M., Ahlawat, Y.S., Chakraborty, N.K., Varma, A. (1993). Detection of the Asian strains of the greening BLO by DNA-DNA hybridisation in Indian orchard trees and Malaysian Diaphorina citri psyllids. In: Proc. 12th Conf. IOCV, 258-63, Univ. Calif., IOCV, Riverside, CA.
Buitendag, C.H., von Broembsen, L.A.(1993). Living with Citrus Greening in South Africa. Proc. 12th Conf. IOCV, 269-273, Univ. Calif., IOCV, Riverside, CA.
Burckhardt, D., Martinez, M. (1989). Note sur la presence au Honduras d’un redoutable ennemi des citrus : Diaphorina citri Kuwayama (Hom. Psylloidea Psyllidae). Bulletin de la Société Entomologique de France 94 : 65-66.
Capoor, S.P. (1963). Decline of citrus trees in India. Bulletin, National Institute of Science India 24: 48-64.
Capoor, S.P., Rao, D.G., Viswanath, S.M. (1967). Diaphorina citri Kuway., a vector of the greening disease of citrus in India. Indian Journal of Agricultural Science 37: 572-576.
Capoor, S.P., Rao, D.G., Viswanath, S.M. (1974). Greening disease of citrus in the Decan Trap Country and its Relationship with the Vector, Diaphorina citri Kuwayama. . Proc. 6th conf. IOCV, 43-49, Univ. Calif., Division Agricultural. Sciences, Riverside, CA .
Catling, H.D. (1969). The bionomics of the South African citrus psylla, Trioza erytreae (Del Guercio) (Homoptera: Psyllidae). III. The influence of extremes of weather on survival. Journal of Entomology Society South Africa 32: 273-290.
Catling, H.D., Atkinson, P.R. (1974). Spread of greening by Trioza erytreae (Del Guercio) in Swaziland. Proc. 6th Conf. IOCV, 33-39, IOCV, Univ. Calif., Riverside, CA.
Cermeli, M., Morales, P., Godoy, P. (2000). Presencia del psilido asiatico de los citricos Diaphorina citri Kuwayama (Hemiptera: Psyllidae) en Venezuela. Boletin de Entomologia Venezolana 15: 235-243.
Chen, J., Deng, X., Liu, S., Pu, X., Li, H., Civerolo, E. (2008). Detection of phytoplasma and Candidatus Liberibacter asiaticus in citrus showing huanglongbing (yellow shoot disease) symptoms in Guangdong, P. R. China. Phytopathology 98: S35. Full paper: Phytopathology, in press.
Chiu, R.J., Tsai, M-Y., and Huang, C-H. (1979). Distribution and retention of tetracyclines in healthy and likubin-infected citrus trees following trunk transfusion. Proc. ROC-US Coop. Sci. Sem. Mycoplasma Dis. Plants (Natl.Sci. Counc. Symp. Ser. No. 1), pp 143-152.
Ciccarone, A. (1957). Il “Giallume” (“Yellow Shoot”) degli agrume in Cina. Revista di Agrumicoltura 2: 45-50.
Coletta-Filho, H.D., Targon, M.L.P.N., Takita, M.A., De Negri Jr, J.D., Pompeu, J., Machado, M.A. (2004). First report of the Causal Agent of Huanglongbing (“Candidatus Liberibacter asiaticus”) in Brazil. Plant Disease 88: 1382.
Conant, P., Hirayama, C., Kumashiro, B.R., Heu, R. A. (2007). Asian citrus psyllid Diaphorina citri in Hawaii and Maui. State of Hawaii Department of Agriculture, New Pest Advisory, Updated February 2007
Davis, M.J., Mondal, S.N., Chen, H., Rogers, M.E., Brlansky, R.H. (2008). Co-cultivation of ‘Candidatus Liberibacter asiaticus’ with Actinobacteria from citrus with huanglongbing. Plant Disease 92: 1547-1550.
Davis, R., Gunua, T., Bellis, G., Grimshaw, J. (2005a). Huanglongbing disease of citrus trees. Pest advisory leaflet no. 45. Plant Protection Service. Secretariat of the Pacific Community. ISSN 1017-6276.
Da Graça, J.V. (1991). Citrus greening disease. Ann. Rev. Phytopathol. 29: 109-36.
Da Graça, J.V., and Korsten, L. (2004). Citrus huanglongbing: review, present status and future strategies. In: S.A.M.H. Naqvi (Ed.). Diseases of Fruits and Vegetables, pp. 229-245. Kluwer Academic Publishers, Dordrecht, The Netherlands.
Ding, F., Wang, G., Yi, G., Zhong, Y., Zeng, J., Zhou, B. (2005). Infection of Wampee and lemon by the citrus huanglongbing pathogen (Candidatrus Liberibacter asiaticus) in China. J. Plant Pathol. 87: 207- 212.
Duan, Y.P., Gottwald, T., Zhou, L.J., Gabriel, D.W. (2008a). First report of dodder transmission of Candidatus Liberibacter asiaticus to tomato (Lycopersicon esculentum). Plant disease 92: 831.
Duan, Y.P., Zhou, L.J., Hall, D., Li, W.B., Liu, L., Gottwald, T. (2008b). Genome sequencing of Candidatus Liberibacter asiaticus, the causal agent of citrus huanglongbing (greening) in Florida. Program and Abstracts Book, Int. Congress Citrus, Wuhan, China, October 2008, abstract 87, p 52.
Etienne, J., Aubert, B. (1980). Biological control of psyllid vectors of greening disease on Reunion Island. Proc. 8th Conf. IOCV, 118-121, IOCV, Univ. Calif., Riverside, CA.
Etienne, J., Burckhardt, D., Grapin, C. (1998). Diaphorina citri (Kuwayama) en Guadeloupe, premier signalement pour les Caraïbes. Bulletin de la Société Entomologique de France 103 : 32.
Fraser, L.R., Singh, D. (1968). Citrus dieback in India – the contribution of greening virus. Proc. 4th Conf. IOCV, 141-144, Univ. Calif., Div. Agr. Sciences, Riverside, CA.
Gao, S., Garnier, M., Bové, J.M. (1993). Production of monoclonal antibodies recognizing most strains of the greening BLO by in vitro immunization with an antigenic protein purified from the BLO. Proc. 12th Conf. IOCV, 244-249. IOCV, Univ. Calif., Riverside, CA.
Garnier, M., Bové, J.M. (1983). Transmission of the organism associated with citrus greening disease from sweet orange to periwinkle by dodder. Phytopathology 73: 1358-1363.
Garnier, M., Bové, J.M. (1993). Citrus greening disease and the greening bacterium. In: Proc. 12th Conf. IOCV, 212-19, IOCV, Univ. Calif., Riverside, CA.
Garnier, M., Bové, J.M. (1996). Distribution of the huanglongbing (greening) liberobacter species in fifteen African ans Asian countries. In: Proc. 13th Conf. IOCV, 388-91, IOCV, Riverside, CA.
Garnier, M., Danel, N., Bové, J.M. (1984a). Aetiology of Citrus Greening Disease. Ann. Microbiology. (Inst. Pasteur) 135A: 169-179.
Garnier, M., Danel, N., Bové, J.M. (1984b). The greening organism is a Gram negative bacterium. Proc 9th Conf. IOCV, 115-124, IOCV, Univ. Calif.,Riverside, CA.
Garnier, M., Gao, S.J., He, Y., Villechanoux, S., Gandar, J., Bové, J.M. (1991). Study of the greening organism (GO) with monoclonal antibodies: serological identification, morphology serotypes and purification of the GO. Proc. 11th Conf. IOCV, 428-435, IOCV, Univ. Calif., Riverside, CA.
Garnier, M., Jagoueix-Eveillard, S., Cronje, P., Le Roux, H., Bové, J.M. (2000). Genomic characterisation of a liberibacter present in an ornamental Rutaceous tree, Calodendrum capense, in the Western Cape province of South Africa. Proposal for a “Candidatus Liberibacter africanus subsp. capensis”. Int. J. Sys. Evol. Microbiol. 50: 2119-2125.
Garnier, M., Jagoueix, S., Toorawa, P., Grisoni, M., Mallessard, R., Dookun, A., Saumtally, S., Autrey, J.C., Bové, J.M. (1996). Both huanglongbing (greening) liberobacter species are present in Mauritius and Reunion. In: Proc. 13th Conf. IOCV, 392-94, IOCV, Univ. Calif., Riverside, CA.
Gatineau, F., Loc, H.T., Tuyen, N.D., Tuan, T.M., Hien, N.T., Truc, N.T.N. (2006). Effects of two insecticide practices on population dynamics of Diaphorina citri and huanglongbing incidence in south Vietnam. p.110. Proc. Huanglongbing–Greening Intern. Workshop, Ribeirão Preto, Brazil.
Gottwald, T.R., Aubert, B., Huang, K.L. (1991). Spatial pattern analysis of citrus greening in Shantou, China.. In R.H. Brlansky, R.F. Lee and L.W. Timmer (eds.) Proc. 11th Conf. IOCV, 421-427, IOCV, Univ. Calif., Riverside, CA.
Gottwald. T.R., Aubert, B., Zhao, X.Y. (1989). Preliminary analysis of citrus greening (Huanglungbin) epidemics in the People's Republic of China and French Reunion Island. Phytopathology, 79:687-693.
Gottwald,.T.R., da Graça, J.V., Bassanezi, R.B. (2007). Citrus huanglongbing: The pathogen, its epidemiology, and impact. Plant Healthy Progress doi:10.1094/PHP-2007-0906-01-RV.
Gottwald, T.R., Irey, M., Gast, T., Parnell, S., Taylor, E., Hilf, M.E. (2009). Spatio-temporal analysis of an HLB epidemic in Florida and implications for future spread. In Proc. 17th Conf. IOCV, Univ. Calif., Riverside (in press).
Gudmestad, N.C., Secor, G.A. (2007). Zebra chip: a new disease of potato. Potato Eyes 19 (issue 1): 1-4.
Halbert, S.E. (2005). The discovery of huanglongbing in Florida. Proceedings of 2nd International Citrus Canker and Huanglongbing Research Workshop, Florida Citrus Mutual, Orlando 2005, H-3.
Halbert, S.E., Manjunath, K.L. (2004). Asian citrus psyllids (Sternorrhycha: Psyllidae) and greening disease of citrus: a literature review and assessmentof risk in Florida. Florida Entomologist 87: 330-53.
Halbert, S.E., Nuñez, C.A. (2004). Distribution of the Asian citrus psyllid, Diaphorina citri Kuwayama (Rhynchota: Psyllidae) in the Caribbean basin. Florida Entomologist 87: 401-2.
Hansen, A. K., Trumble, J. T., Stouthammer, R., Paine, T. D. (2008). A new Huanglongbing species, “Candidatus Liberibacter Psyllaurous”, found to infect tomato and potato, is vectored by the psyllid Bactericera cockerelli (Sulc). Appl. Environ. Microbiol. 74: 5862-5865.
Hocquellet, A., Toorawa, P., Bové, J.M., Garnier, M. (1999). Detection and identification of the two “Candidatus Liberobacter sp.” Associated with citrus huanglongbing by PCR amplification of ribosomal protein genes of the beta operon. Mol. Cell. Probes 13: 373-379.
Hung, T.H., Wu, M.L., Su, H.J. (2000). Identification of alternative hosts of the fastidious bacterium causing citrus greening disease. Journal of Phytopathology 148: 231.
Irey, M.S., Gast, T., Gottwald, T.R. (2006). Comparison of visual assessment and polymerase chain reaction assay testing to estimate the incidence of the Huanglongbing pathogen in commercial Florida citrus. Proc. Florida State Hortic. Soc., 119: 89-93.
Jagoueix, S., Bové, J.M., Garnier, M. (1994). The phloem-limited bacterium of greening disease of citrus is a member of the α subdivision of the Proteobacteria. International Journal of Systematic Bacteriology 44: 397-86.
Jagoueix, S., Bové, J.M., Garnier, M. (1996). PCR detection of the two ‘Candidatus’ liberobacter species associated with greening disease of citrus. Molecular and Cellular Probes 10: 43-50.
Jagoueix, S., Bové, J.M., Garnier, M. (1997). Comparison of the 16S/23S Ribosomal Intergenic Regions of “Candidatus Liberobacter asiaticum” and “Candidatus Liberobacter africanum”, the two species associated with Citrus Huanglongbing (Greening) Disease. Int. Journal of Systematic Bacteriology 47: 224-227..
Laflèche, D., Bové, J.M. (1970a). Structures de type mycoplasme dans les feuilles d’orangers atteints de la maladie du greening. C.R.Acad. Sci. Paris, 270: 1915-17..
Laflèche, D., Bové, J.M. (1970b). Mycoplasmes dans les agrumes atteints de “greening”, de “stubborn” ou de maladies similaires. Fruits 25 : 455-465.
Li, W., Hartung, J.S., Levy, L. (2006). Quantitative real time PCR for detection and identification of Candidatus Liberibacter species associated with citrus huanglongbing. J. Microbiol. Methods 66:104-115.
Li, W., Hartung, J.S., Levy, L. (2007). Evaluation of DNA amplification methods for improved detection of “Candidatus Liberibacter species” associated with citrus huanglongbing. Plant Disease 91: 51-58.
Liefting, L. W., Sutherland, P. W., Paice, L. I., Weir, B. S., Clover, G. R. G. (2009a). A new “Candidatus Liberibacter” species associated with diseases of solanaceous crops. Plant disease (in press).
Liefting, L. W., Weir, B. S., Pennycook, S. R., Clover, G. R. G. (2009b). ‘Candidatus Liberibacter solanacearum’, a liberibacter associated with plants in the family Solanaceae. Int. J. Syst. Evol. Microbiol. (in press).
Lin, Hong, Doddapaneni, H., Munyaneza, J., Civerolo, E., Venkatesan, S., Buchman, J., Stenger, D. (2009). Molecular characterization and phylogenetic analysis of 16S rRNA from “Candidatus Liberibacter” strains associated with Zebra chip disease of potato (Solanum tuberosum L.) and the potato psyllid (Bactericera cockerelli Sulc). J. Plant Path. (in press).
Lin, Kung Hsiang. (1956a). Yellow shoot of citrus (in Chinese). Acta Phytopathologica Sinica 2: 1-12.
Lin, Kung Hsiang. (1956b). Etiological studies of yellow shoot of citrus (in Chinese). 42. Acta Phytopathologica Sinica 2: 12-42.
Lin, C.K. (1963). Notes on citrus yellow shoot disease. Acta Phytophylact. Sinica. 2: 243-251.
Lopes, S.A., Bertolini, E., Frare, G.F., Martins, E.C., Wulff, N.A., Teixeira, D.C., Fernandes, N.G., Cambra, M. (2009a). Graft Transmission Efficiencies and Multiplication of Candidatus Liberibacter americanus and Ca. Liberibacter asiaticus in Citrus Plants. Phytopathology (in press).
Lopes, S.A., Frare, G.F. (2008). Graft transmission and cultivar reaction of citrus to ‘Candidatus Liberibacter americanus. Plant Disease 92: 21-24.
Lopes, S.A., Frare, G.F., Bertolini, E., Cambra, M., Fernandes N.G., Ayres, A.J., Marin, D.R., Bové, J. M. (2009b). Liberibacters associated with citrus huanglongbing in Brazil: Candidatus Liberibacter asiaticus is heat tolerant, Candidatus Liberibacter americanus is heat sensitive. Plant Disease (in press).
Lopes, S.A., Frare, G.F., Yamamoto, P.T., Ayres, A.J., Barbosa, J.C. (2007). Ineffectiveness of pruning to control citrus huanglongbing caused by Candidatus Liberibacter americanus. Eur. J. Plant Pathol. 119: 463-468.
Lopes, S.A., Martins, E.C., Frare, G.F. (2005). Deteção de Candidatus Liberibacter americanus em Murraya paniculata. Summa Phytopathologica 31: 48-49.
Lopes, S.A., Martins, E.C., Frare, G.F. (2006). Deteção de Candidatus Liberibacter asiaticus em Murraya paniculata. Fitopatologia Brasileira 31: 303.
Lou, B. H., Zhou, C.Y., Zhao, X. Y., Li, Z. G., Xu, M., Liu, J. X., Zhou, Y., Tang, K. S. (2008). Primary study on species and intraspecific differentiations of HLB pathogens in eight provinces of China. Program and Abstracts book, 11th international citrus congress, p. 232, abstract P333.
Luis Pantoja, M., Collazo Cordero, C., Llauger Riveron, R., Peña Barzaga, L., Lopez Hernandez, D., Blanco, E., Casi, J.C., Batista Le Riverend, L., Tanaka, F.A.O., Salaroli, R.B., Martins, E., Teixeira, D., Kitajima, E., Ayres, J., Bové, J.M., Perez, J.L., Cueto, J.R. (2008). Huanglongbing in Cuban Citriculture. Book of Program and Abstracts, 11th International Citrus Congress, Wuhan, China, 26-30 Oct. 2008, Abstract # 84, p. 50-51.
Manjunath, K., Halbert, S., Ramadugu, C., Webb, S., Lee, R. (2008). Detection of Candidatus Liberibacter asiaticus in Diaphorina citri and its importance in the management of citrus huanglongbing in Florida. Phytopathology 98; 387-396.
Martinez, A.L., Wallace, J.M. (1967). Citrus leaf mottle-yellows disease in the Philippines and transmission of the causal virus by a psyllid, Diaphorina citri. Plant Disease Reporter 51: 692-695.
Martinez, A.L., Wallace, J.M. (1968). Studies on leaf-mottle-yellows disease of citrus in the Philippines. Proc. 4th Conf. IOCV, University Florida Press, Gainesville 1968, 167-176.
McClean, A.P.D., Oberholzer, P.C.J. (1965a). Greening disease of sweet orange: evidence that it is caused by a transmissible virus. South Africa Journal of Agricultural Science 8: 253-276.
McClean, A.P.D., Oberholzer, P.C.J. (1965b). Citrus psylla, a vector of the greening disease of sweet orange. South Africa Journal of Agricultural Science 8: 297-298.
McClean, A.P.D., Schwarz, R.E. (1970). Greening or blotchy-mottle disease of citrus. Phytophylactica 2: 177-194.
Moll, J.N., Martin, M.M. (1974). Comparison of the organism causing greening disease with several plant pathogenic Gram negative bacteria, rickettsia-like organisms and mycoplasma-like organisms. Proceedings of Conference Les Mycoplasmes/Mycoplasmas, INSERM 33 : 89-96.
Moll, J.N., Van Vuuren, S.P. (1977). Greening disease in Africa. Proc. Int. Soc. Citricult. 3; 903-912.
Moll, J.N., van Vuuren, S.P., Milne, D.L. (1980). Greening disease, the South African situation. Proc. 8th Conf. IOCV, 109-117, IOCV, Univ. Calif., Riverside, CA.
Murray, R.G.E., Schleifer, K.H. (1994). Taxonomic notes: a proposal for recording the properties of putative taxa of prokaryotes. Int. J. Syst. Bacteriol.. 44: 174-176.
Murray, M.G., and Thompson, W.F. (1980). Rapid isolation of high molecular weight plant DNA. Nucleic Acids Research, 8: 4321-5.
Noronha Jr, N. C., Bento, J. M. S., Parra, J. R. P. (2008). Guava and citrus plant volatiles. Fundecitrus workshop on HLB-resistant citrus. Araraquara, February 2008.
Phahladira, M.N.B. (2008). Identification of alternative hosts to citrus of "Candidatus Liberibacter africanus" amongst indigenous Rutaceae of South Africa. MSc. study in progress., University of Pretoria, Pretoria, South Africa.
Planet, P., Jagoueix, S., Bové, J.M., Garnier, M. (1995). Detection and characterization of the African citrus greening liberobacter by amplification, cloning and sequencing of the rplKAJL-rpoBC operon. Current Microbiology 30: 137-141.
Reinking, O.A. (1919). Diseases of economic plants in southern China. Philippines Agric. 8: 109-135.
Roistacher, C.N. (1996). The economics of living with citrus diseases: huanglongbing (greening) in Thailand.. Proc. 13th Conf. IOCV, 279-285, IOCV, Univ. Calif., Riverside, CA.
Saglio, P., Laflèche, D., Bonissol, C., Bové, J.M. (1971). Isolement, culture et observation au microscope électronique des structures de type mycoplasme associés à la maladie du stubborn des agrumes et leur comparaison avec les structures observées dans le ces de la maladie du greening des agrumes. Phys. Veg. 9 : 569-82.
Salibe, A.A., Cortez, R.E. (1968). Leaf mottling, a serious virus disease of citrus in the Philippines. In: University Florida (ed.). Proc. 4th Conf. IOCV, 131-136, Univ. Florida Press, Gainesville, FL.
Schwarz, R.E., Knorr, L.C., Prommintara, M. (1973). Presence of citrus greening and its psylla vector in Thailand. FAO Plant Prot. Bull. 21: 132-38.
Schwarz, R.E., van Vuuren, S.P. (1971). Decrease in fruit greening of sweet orange by trunk injections of tetracyclines. Plant Dis. Reptr. 55: 747-750. .
Schwarz, R.E., Moll, J.N., van Vuuren, S.P. (1974). Control of citrus greening and its psylla vector by trunk injections of tetracyclines and insecticides. Proc. 6th Conf. IOCV, 26-29, Univ. Calif., Div. Agr. Sciences, Riverside, CA.
Sechler, A., Schuenzel, E.L., Cooke, P., Dannua, S., Thaveechai, N, Postnikova, E., Stone, A.L., Schneider, W.L., Aarmsteegt, V.D., Schaad, N.W. (2009). Cultivation of ‘Candidatus Liberibacter asiaticus’ and ‘Candidatus Liberibacter americanus’ associated with huanglongbing. Phytopathology (in press).
Su, H.J., Chang, S.C. (1976). The responses of likubin pathogen to antibiotics and heat therapy. Proc. 6th Conf. IOCV, 27-34, Univ. Calif., Div. Agr. Sciences, Riverside, CA.
Subandiyah, S., Iwanami, T., Tsuyumu, S., Ikki, H. (2000). Comparison of 16S RNA and 16S23S intergenic region sequences among citrus greening organisms in Asia. Plant Dis. 84: 15-18.
Supriyanto, A., Whittle, A.M. (1991). Citrus rehabilitation in Indonesia. Proc. 11th Conf. IOCV, 409-413, IOCV, Univ. Calif., Riverside, CA.
Texeira, D.C., Ayres, A.J., Kitajima, E.W., Tanaka, F.A.O., Danet, J.L.,. Jagoueix-Eveillard, S, Saillard, C., Bové, J.M. (2005a). First Report of a Huanglongbing-like Disease of Citrus in Sao Paulo State, Brazil, and Association of a New liberibacter Species, “Candidatus Liberibacter americanus”, with the Disease. Plant Disease 89: 107.
Teixeira, D. C., Danet, J.L.,, Eveillard, S., Martins, E.C.,. de Jesus Junior, W.C, Yamamoto, P.T., Lopes, S.A., Bassanezi, R.B., Ayres, A.J., Saillard, C., Bové, J.M. (2005b). Citrus huanglongbing in São Paulo State, Brazil: PCR detection of the “Candidatus” Liberibacter species associated with the disease. Molecular and Cellular Probes, 19; 173-179.
Teixeira, D.C., Lopes, S.A.,, Yamamoto, P.T., Eveillard, S., Martins, E.C., de Jesus Junior, W.C., Bassanezi, R.B., Ayres, A.J., Danet, J.L., Saillard, C., Bové, J.M. 2005c. PCR detection of the two liberibacter species associated with citrus huanglongbing (HLB) in São Paulo State, Brazil, Proc. 16th conf. IOCV, 432-438, IOCV, Riverside, CA.
Teixeira, D. C., Saillard, C., Eveillard, S., Danet, J.L., Ayres, A.J., Bové, J.M. (2005d). Candidatus Liberibacter americanus sp. nov., associated with citrus huanglongbing (greening disease) in São Paulo State, Brazil. Intern. J. Syst. Evol. Microbiol. 55: 1857-1862.
Teixeira, D.C., Saillard, C., Eveillard S., , Danet J.L., , Ayres, A.J., Bové, J.M. (2005e). A new liberibacter species, Candidatus Liberibacter americanus sp. nov., is associated with citrus huanglongbing (greening disease) in São Paulo state, Brazil. Proc. 16th conf. IOCV, 325-340, IOCV, Riverside, CA.
Teixeira, D. C., Eveillard, S., Sirang-Pugnet, P., Wulff, N.A., Saill ard, C., Ayres, A.J., Bové, J.M. (2008a). The tufB-secE-nusG-rplKAJL-rpoB gene cluster of the liberibacters: sequence comparisons, phylogeny and speciation. Int. J. Syst. Evol. Microbiol. 58: 1414-1421.
Teixeira, D. C., Saillard, C., Couture, C., Martins, E.C., Wulff, N.A., Eveillard-Jagoueix, S., Yamamoto, P.T., Ayres, A.J., Bové, J.M. (2008b). Distribution and quantification of Candidatus Liberibacter americanus, agent of huanglongbing disease of citrus in São Paulo state, Brazil, in leaves of an affected sweet orange tree as determined by PCR. Mol. Cell. Probes 22: 139-150.
Teixeira, D. C., Wulff, N. A., Martins, E. C., Kitajima, E. W., Bassanezi, R., Ayres, A. J., Eveillard, S., Saillard, C., Bové, J. M. (2008c). A phytoplasma closely related to the Pigeon pea witches’ broom phytoplasma (16Sr IX) is associated with citrus huanglongbing symptoms in the State of São Paulo, Brazil. Phytopathology 98: 977-984.
Vaccaro, N. C. (1994). Comportamiento y diffusion de la “Chicharrita de los Citrus” Diaphorina citri en la zona citrica de Entre Rios. Información Citricota. CITRUS vol.2 – INTA - E.E.A.
Van der Merwe, A.J., Andersen, F.G. (1937). Chromium and manganese toxicity. Is it important in Transvaal citrus greening? Farming South Africa 12: 439-440.
Villalobos, W., Hollis, D., Godoy, C., Rivera, C. (2005). First report of Diaphorina citri (Hemiptera: Psyllidae) in Costa Rica. Insecta Mundi 19: 191-192.
Villechanoux, S., Garnier, M., Renaudin, J., Bové, J.M. (1992). Detection of several strains of the bacterium-like organism of citrus greening disease by DNA probes. Current Microbiology 24: 89-95.
Villechanoux, S., Garnier, M., Laigret, F., Renaudin, J., Bové, J.M. (1993). The genome of the non-cultured, bacterial-like organism associated with citrus greening disease contains the nusG-rplKAJL-rpoBC gene cluster and the gene for a bacteriophage type DNA polymerase. Current Microbiology 26: 161-166.
Wang, Z.,.Yin,Y., Hu, H., Yuan, Q., Peng, G., Xia, Y. (2006). Development and application of molecular-based diagnosis for ‘Candidatus Liberibactre asiaticus’, the causal pathogen of citrus huanglongbing. Plant Pathology 55: 630-638.
Weisburg, W.G., Barns, S.M., Pelletier, D.A., Lane, D.J. (1991). 16S ribosomal DNA amplification for phylogenetic study. Journal of Bacteriology 173: 697-703.
Wulff, N.A., Eveillard, S., Foissac, X., Ayres, A.J., Bové, J.M. (2009). Ribosomal RNA operons and Genome size of Candidatus Liberibacter americanus, a bacterium associated with citrus huanglongbing in Brazil. Int. J. Syst. Evol. Microbiol. (in press).
Xu, C.F., Xia, Y.H., Li, K.B., Ke, C. (1988). Further study of the transmission of citrus huanglongbin by a psyllid, Diaphorina citri Kuwayama. Proc. 10th conf. IOCV, 243-248, IOCV, Univ. Calif., Riverside, CA.
Yamamoto, P. T., Felippe, M. R., L. F. Garbim et al., (2006). Diaphorina citri (Kuwayama) (Hemiptera: Psyllidae): vector of the bacterium Candidatus Liberibacter americanus. Page 96 in: Proc. of the Huanglongbing-Greening Intl. Workshop, Ribeirão Preto, SP, Brazil.
Zhou, L.J., Gabriel, D.W., Duan, Y.P., Halbert, S.E., Dixon, W.N. (2007). First report of dodder transmission of huanglongbing from naturally infected Murraya paniculata to citrus. Plant Disease 91:227.
Zhou, L., Duan, Y., Gabriel, D., Gottwald, T.R. (2008a). Seed transmission of Candidatus Liberibacter asiaticus in periwinkle and dodder resulted in low bacterial titer and very mild disease in periwinkle. Phytopathology 98: S181.
Zhou, L.J., Benyon, L.S., Powell, C.A., Gottwald, T., Duan, Y.P. (2008b). Improved detection of low-titer, non-lethal, seed-transmitted Candidatus Liberibacter asiaticus in citrus, periwinkle and dodder using nested PCR. Proc. Int. Conf. HLB., Dec. 2008, Orlando, Abstract 3.2, p112-113.
.
Prepared (1975) and revised (1980) by
R. E. Schwarz & J. M. Bové
I. N. I. A. Laboratoire de Biologie
Montecarlo, Misiones cellulaire et moléculaire
(ARGENTINA) (FRANCE)
Revised September 2008 and January 2009
by J.M. Bové
IBVM, Laboratoire de Biologie Cellulaire and Moleculaire,
I.N.R.A./ Université Victor Ségalen Bordeaux 2,
Centre INRA de Bordeaux,
71, Ave. E. Bourleau,
33883 Villenave d’Ornon
FRANCE
Click on any image to see it larger.
PHOTOS |
LEGENDS AND AUTHORS |
|
|---|---|---|
|
Map of China, South of the Yang Tse river. Notice “Chaozhou” (in red rectangle), where HLB is supposed to have started in China - J. M. Bové |
||
|
Professor Lin Kung Hsiang: first transmission of the HLB agent by graft-inoculation (1956). Professor Antonio Ciccarone: reported Lin’s work in Italian citrus journal (1957) – J. M. Bové |
||
|
Distribution of African HLB liberibacter (red circles) and Asian HLB liberibacters (blue circles) as known in 2003 – J. M. Bové |
||
|
Distribution of African, Asian, and American HLB liberibacters and American and Asian HLB phytoplasmas as known in December 2008 – J. M. Bové |
||
|
Mediterranean Region is free of HLB, but HLB psyllid-vectors are not far - J. M. Bové |
||
|
Australia and New Zealand (NZ) are free of HLB and citrus liberibacters, but NZ and North America have the potato liberibacter (pink rectangle) - J. M. Bové |
||
|
Characteristics of African, Asian, and American HLB - J. M. Bové |
||
|
Left: view of Guilin-Yangshuo region (Guangxi province, China). Right: sweet orange tree affected by Huang Long Bing or Yellow Shoot Disease (Yangshuo region) - J. M. Bové |
||
|
Sweet orange orchard with trees affected by Huang Long Bing or Yellow Shoot Disease (Yangshuo region) - J. M. Bové |
||
|
Young sweet orange tree with two adjacent yellow shoots (São Paulo State, Brazil) - J. M. Bové |
||
|
Young sweet orange tree with several yellow shoots (São Paulo State, Brazil) - J. M. Bové |
||
|
Adult sweet orange tree with several yellow shoots (São Paulo State, Brazil) - J. M. Bové |
||
|
Young, HLB-affected sweet orange tree with: (i) symptomatic yellow/pale green branch, (ii) yellow shoots, (iii) symptomless dark-green branch (São Paulo State, Brazil) - J. M. Bové |
||
|
Sweet orange tree with characteristic Yellow Branch J. M. Bové |
||
|
Normal, symptomless sweet orange tree near affected tree of photo HLB. 012 (Rustenburg area, South Africa) - J. M. Bové |
||
|
Fully affected sweet orange tree. Trees of photos HLB. 012, 013, and 014 are from the same Rustenburg orchard - J. M. Bové |
||
|
1: symptomless sweet orange tree. 2: tree with yellow shoots in upper part, but lower part is essentially symptomless (Araraquara area, São Paulo State, Brazil) - J. M. Bové |
||
|
3: tree similar to tree 2 of photo HLB. 015, except for symptoms starting in lower part. 4: fully affected tree. Trees 1 to 4 are from the same Araraquara orchard - J. M. Bové |
||
|
Severely affected sweet orange tree in the region of high HLB incidence (Araraquara area) in São Paulo State, Brazil - J. M. Bové |
||
|
Nine-year-old sweet orange trees in an orchard severely affected by HLB in January 2005. In October 1999, the orchard was essentially symptomless, symptomatic trees (7%) having been removed. In the absence of any HLB-control measures from Oct. 1999 to Jan. 2005, it took only 5 years for the orchard to be completely destroyed. (Behai, Guangxi province, China) See HLB. 001 for location of Behai on the southern coast of China. |
||
|
Young Valencia sweet orange tree fully affected by HLB (Ciego de Avila, Cuba) – J. M. Bové |
||
|
Young Valencia sweet orange tree with severely affected yellow branch (white circle) in contrast to most of the tree, much less affected. Compare with tree of photo HLB. 019, from the same orchard (Ciego de Avila, Cuba) – J. M. Bové |
||
|
Young Valencia sweet orange tree similar to tree of photo HLB. 020, but from Jagüey Grande region (Cuba) - J. M. Bové |
||
|
Also from the Jagüey Grande orchard of photo HLB. 021: young Valencia sweet orange tree severely affected, except for one, almost symptomless, green sector (yellow circle) - J. M. Bové |
||
|
Close-up of the green sector of tree on photo HLB. 022: leaves are essentially symptomless and green - J. M. Bové |
||
|
Close-up of an affected sector of tree on photo HLB. 022: many leaves are yellow. Compare with photo HLB. 023 - J. M. Bové |
||
|
HLB-affected seedling mandarin trees of local, Nepalese variety. In Nepal, all commercial citrus trees are seedling trees (Lamjung, Nepal) - J. M. Bové |
||
|
Close-up of HLB-affected seedling mandarin tree. Some branches have yellow leaves; leaves of other branches are still green (Lamjung, Nepal) - J. M. Bové |
||
|
Severely affected seedling mandarin tree: most leaves are yellow. Notice defoliation. Compare with tree of photo HLB. 026 (Lamjung, Nepal) - J. M. Bové |
||
|
Trees 1 and 2 (photo HLB. 028), and 3 and 4 (photo HLB. 029) are seedling mandarin trees from the same orchard in the Punakha valley, Bhutan, where HLB was first seen in the early 2000s . Like in Nepal, all commercial trees are seedling trees of a local variety. 1: green, symptomless tree. 2: HLB-affected tree with green and yellow sectors (Punakha valley, Bhutan) - J. M. Bové |
||
|
3: HLB-affected tree with green and yellow sectors, similar to tree 2 of photo HLB. 028. 4: HLB-affected tree, almost entirely yellow. (Punakha valley, Bhutan) - J. M. Bové |
||
|
Young Dancy tangerine trees of photo HLB. 030, 031, and 032 are from an orchard in Ciego de Avila, Cuba, severely affected by HLB. Photo HLB. 030 shows least affected tree - J. M. Bové |
||
|
Affected Dancy tangerine tree with leaves, still green, in top of tree (Ciego de Avila, Cuba) - J. M. Bové |
||
|
Affected Dancy tangerine tree with some defoliation (Ciego de Avila, Cuba) - J. M. Bové |
||
|
HLB-affected Murcott tangor tree. Notice several yellow sectors (São Paulo state, Brazil) – J. M. Bové |
||
|
Green, symptomless Marsh grapefruit tree in a young, HLB-affected orchard (Ceiba del Agua, Cuba) – J. M. Bové |
||
|
HLB-affected Marsh grapefruit tree from orchard of photo HLB 034. The tree has still some green leaves (Ceiba del Agua, Cuba) – J. M. Bové |
||
|
HLB-affected Marsh grapefruit tree from orchard of photo HLB 034. Practically all leaves have turned yellow (Ceiba del Agua, Cuba) – J. M. Bové |
||
|
Normal, uniformly green sweet orange leaves – J. M. Bové |
||
|
Chimeric Etrog citron leaves. These leaves have different shades of green colour: they show “mottle”. However, the various shades of green are separated from each other by sharp boundaries. In other words, there is NO merging of one shade into another: the mottle is NOT ”blotchy” – J. M. Bové |
||
|
Sour orange leaves showing ‘Petri’s variegation or mottle” in Tunisia. Like the Etrog citron mottle of photo HLB. 038, the sour orange mottle is NOT “blotchy” – J. M. Bové |
||
|
HLB-affected sweet orange leaf showing characteristic “blotchy” mottle. The various shades of green are not separated from each other by sharp boundaries; they do merge into each other (São Paulo State, Brazil) – J. M. Bové |
||
|
Shoots with HLB-affected sweet orange leaves showing characteristic “blotchy” mottle (Behai, Guangxi province, China) – J. M. Bové |
||
|
HLB-affected sweet orange leaves showing characteristic “blotchy” mottle (Behai, Guangxi province, China) – J. M. Bové |
||
|
Shoots with HLB-affected sweet orange leaves showing characteristic “blotchy” mottle in South Africa, very similar to the “blotchy” mottle in China: compare photo HLB. 041 with HLB. 43 (South Africa) – J. M. Bové |
||
|
Characteristic HLB “blotchy” mottle on sweet orange leaves (South Africa) – J. M. Bové |
||
|
HLB-affected sweet orange leaf illustrating “blotchy” mottle (South Africa) – J. M. Bové |
||
|
Sweet orange leaves with blotchy mottle In São Paulo State, Brazil – J. M. Bové |
||
|
Characteristic blotchy mottle on sweet orange leaves (São Paulo State, Brazil) – J. M. Bové |
||
|
Characteristic blotchy mottle on additional sweet orange leaves (São Paulo State, Brazil) – J. M. Bové |
||
|
Sweet orange leaves with blotchy mottle in Florida, USA – J. M. Bové |
||
|
Additional sweet orange leaves with blotchy mottle in Florida, USA – J. M. Bové |
||
|
Sweet orange leaves with blotchy mottle in La Habana, Cuba – J. M. Bové |
||
|
Shoot with blotchy mottle leaves from HLB-affected seedling tree of local sweet orange variety (Dhankuta, Nepal) – J. M. Bové |
||
|
Right: normal sweet orange leaf. Left: several blotchy mottle leaves from HLB-affected sweet orange tree from Madagascar (altitude: ~1000m) – J. M. Bové |
||
|
Center: sweet orange leaf showing zinc deficiency symptoms; the right half leaf is more or less symmetrical of the left half leaf. Left and right: leaves with blotchy mottle showing no symmetry– J. M. Bové |
||
|
Sweet orange leaves with zinc deficiency symptoms (São Paulo State, Brazil) – J. M. Bové |
||
|
Sweet orange leaves with zinc deficiency symptoms from mild to severe (São Paulo State, Brazil) – J. M. Bové |
||
|
Severe zinc deficiency symptoms on sweet orange leaves. Left: leaves almost totally yellow. Right: yellow leaf with green “islands”, characteristic of severe zinc deficiency (São Paulo State, Brazil) – J. M. Bové |
||
|
Comparison between sweet orange leaves with zinc or manganese deficiencies. Right: three leaves with zinc deficiency symptoms, characterized by yellow interveinal colour. Left: leaf with manganese deficiency symptom, characterized by pale green interveinal colour (Southern Tunisia) – J. M. Bové |
||
|
Sweet orange leaves showing magnesium deficiency symptoms, with characteristic central green triangle (São Paulo State, Brazil) – J. M. Bové |
||
|
Sweet orange leaves from HLB-affected tree. Upper row leaves: the blotchy mottle pattern does not show up well. Lower leaves: essentially yellow leaves with zinc deficiency patterns; third leaf from right has good blotchy mottle (Behai, China) – J. M. Bové |
||
|
Sweet orange leaves with blotchy mottle showing in addition corky veins (white arrows) (São Paulo State, Brazil) – J. M. Bové |
||
|
Sweet orange leaves with blotchy mottle, prominent midrib, and corky lateral veins (São Paulo State, Brazil) – J. M. Bové |
||
|
Blotchy mottle on grapefruit leaves from HLB-affected tree (La Habana, Cuba) – J. M. Bové |
||
|
Blotchy mottle leaves from pumelo, grapefruit, citron, and Rough lemon trees (Puna, India) – J. M. Bové |
||
|
Blotchy mottle on Citrus macrophylla leaves (Jagüey Grand, Cuba) – J. M. Bové |
||
|
Blotchy mottle on Citrus volkameriana leaves (Jagüey Grand, Cuba) – J. M. Bové |
||
|
Blotchy mottle on sour orange leaves (La Habana, Cuba) – J. M. Bové |
||
|
Blotchy mottle on lemon leaves is often characterized by large dark green patches, as shown on this photo (Nelspruit, South Africa)– J. M. Bové |
||
|
Blotchy mottle on lemon leaves (Nelspruit, South Africa) – J. M. Bové |
||
|
Blotchy mottle on lemon leaves (Robertson, South Africa) – J. M. Bové |
||
|
Blotchy mottle on lemon leaves. Notice, in lower right corner, leaves with “bumps” induced by development of Trioza erytreae nymphs (Nelspruit, South Africa) – J. M. Bové |
||
|
Normal, uniformly green lemon leaves (Nelspruit, South Africa) – J. M. Bové |
||
|
Lime (Citrus aurantifolia) leaves with blotchy mottle of the “lemon type”, characterized by large dark green patches (Ciego de Avila, Cuba) – J. M. Bové |
||
|
Lime (Citrus aurantifolia) leaves with blotchy mottle of the “lemon type”, characterized by large dark green patches (Patan, Nepal) – J. M. Bové |
||
|
Leaves of Tahiti lime (Citrus latifolia) also show the “lemon type” of blotchy mottle (Araraquara, São Paulo State, Brazil) – J. M. Bové |
||
|
“Lemon type” blotchy mottle is very frequent on leaves of Tahiti lime trees affected by HLB in Cuba (Jagüey Grande, Cuba) – J. M. Bové |
||
|
Clementine leaves. Upper row: normal leaves, uniformly green. Lower row: leaves with classic blotchy mottle (South Africa) – J. M. Bové |
||
|
Classic blotchy mottle on Dancy tangerine leaves (Ciego de Avila, Cuba) – J. M. Bové |
||
|
Blotchy mottle on Minneola tangelo leaves (Bhutan) – J. M. Bové |
||
|
After the “sweet orange type” of blotchy mottle and “the lemon type” of blotchy mottle, some mandarins, such as the “Coorg” mandarin, show a third type of blotchy mottle, “mandarin type” mottle characterized by very small colour patches or, rather, colour “touches”, as shown in particular by the circled leaf. The two leaves on the far right show zinc / manganese deficiency symptoms. As shown on the left, HLB-affected mandarin leaves from field trees tend to be more twisted than HLB-affected sweet orange leaves (Coorg region, Karnataka State, India) – J. M. Bové |
||
|
Excellent “mandarin type” blotchy mottle on leaves of local Nepalese variety. Notice twisted leaves (Nepal) – J. M. Bové |
||
|
“Mandarin type” blotchy mottle on leaves of local Nepalese variety. In lower row, first and third leaves from left show some zinc deficiency symptoms at the distal half (Nepal) – J. M. Bové |
||
|
Upper row leaves: “mandarin type” blotchy mottle; first and fourth leaves from left show some “sweet orange type” mottle. Lower row leaves show severe zinc deficiency symptoms (Coorg region, Karnataka State, India) – J. M. Bové |
||
|
Blotchy mottle and zinc deficiency symptoms on leaves of local Nepalese variety (Nepal) – J. M. Bové |
||
|
Normal sweet orange (left) and sweet orange affected by HLB-induced “colour inversion” (right) (São Paulo State, Brazil) – J. M. Bové |
||
|
Left: sweet oranges from South Africa, Swaziland, and Brazil showing colour inversion. Right: normal fruits in Brazil – J. M. Bové |
||
|
Symptoms shown by “Greening-affected” sweet oranges from South Africa are often due to colour inversion (South Africa) – J. M. Bové |
||
|
Pressing with the thumb a sweet orange affected by “greening”, i.e. colour inversion, induces a silvery “finger mark”. Frequently observed in South Africa, it is also seen in São Paulo State, Brazil – J. M. Bové |
||
|
When fruits are still green, removing the peduncle of a sweet orange reveals the circular, peduncle-insertion zone. This zone is stained orange on HLB-affected fruit as shown on this photo, while the equivalent zone on normal fruit is pale-green, as shown on photo HLB. 089. The orange coloration seen at the pedoncular half of the fruit as the result of colour inversion, actually starts at the circular, peduncle-insertion zone, and can be seen only by removing the peduncle (fruits 1, 2, and 3). Later, the orange coloration extends out of this zone and begins to affect the fruit around the peduncle (fruits 4 and 5) (Brazil, Cuba, South Africa) – J. M. Bové |
||
|
On normal fruit, the circular peduncle-insertion zone is pale-green, not only when the fruit is still green, but also when the fruit has turned orange (São Paulo State, Brazil) – J. M. Bové |
||
|
The orange stain, characteristic of the peduncle-insertion zone (upper fruit), is not only located at the surface of this zone, but can also be seen to stain the vascular bundles when the fruit is cut, perpendicular to the fruit-axis, a few millimetres below the peduncle-insertion zone (middle fruit). The lower fruit has been cut in half through the fruit axis, and shows the orange stained vascular bundles (São Paulo State, Brazil) – J. M. Bové |
||
|
The two halves of an HLB-affected sweet orange cut in half through the fruit axis. Notice fruit lopsideness, brownish-black aborted seeds, orange stained vascular bundles at peduncular half of fruit, and difference in colour of vesicles in the large fruit sector and the small fruit sector (China) – J. M. Bové |
||
|
Lopsided, HLB-affected sweet oranges are smaller than normal oranges (China) – J. M. Bové |
||
|
The albedo around the peduncle-insertion zone is often thickened, resulting in a depressed peduncle-insertion zone (São Paulo State) – J. M. Bové |
||
|
Fruit-drop is characteristic of HLB-affected sweet orange trees (South Africa) – J. M. Bové |
||
|
Fruit-drop is characteristic of HLB-affected sweet orange trees (Cuba) – J. M. Bové |
||
|
Spectacular colour inversion and seed abortion on mandarins (China, Indonesia, Thailand) – J. M. Bové |
||
|
Spectacular colour inversion on mandarins (Nepal) – J. M. Bové |
||
|
A : HLB-affected Murcott tangor fruits showing lopsideness, colour inversion, orange-stained vascular bundles at peduncular end of fruit axis, orange-stained peduncle-insertion site, thickened albedo around peduncle-insertion site, depressed peduncle- insertion, and small, aborted seeds. B: Normal fruit is much larger than HLB-affected fruit. Peduncle-insertion site is pale-green (São Paulo State, Brazil) – J. M. Bové |
||
|
HLB-affected pumelos: small size, lopsidedness, aborted seeds, orange-stained vascular bundles at pedoncular fruit axis (Cambodia) – J. M. Bové |
||
|
Electron micrographs by Dominique Laflèche and J. M. Bové (1970) showing the presence of bacteria in the sieve tubes of HLB-affected, sweet orange seedlings graft-inoculated with infected citrus tissues from South Africa, Reunion island and India (Versailles, France) – D. Laflèche and J. M. Bové |
||
|
Schematic representation of the envelope system of Gram positive bacteria, Gram negative bacteria, and mycoplasmas – J. M. Bové |
||
|
Electron micrographs by Monique Garnier and J. M. Bové (1977) showing that the bacterium associated with HLB has a triple- layered cytoplasmic membrane as well as a triple-layered cell- wall membrane. Mycoplasmas lack cell-walls and are surrounded only by a cytoplasmic membrane (Bordeaux, France) - M. Garnier and J. M. Bové |
||
|
Electron micrographs by Monique Garnier and J. M. Bové (1984) showing that the HLB-associated bacterium has a peptidoglycane layer between the inner cytoplasmic membrane and the outer cell-wall membrane, and is therefore a Gram negative bacterium, later characterized as Candidatus Liberibacter spp. (Bordeaux, France) - M. Garnier and J. M. Bové |
||
|
Electron micrograph of Candidatus Liberibacter americanus in a sieve tube of an experimental, HLB-affected periwinkle plant (Bordeaux, France) – B. Gelie and J. M. Bové |
||
|
In addition to liberibacters, it has been found in São Paulo State, Brazil, and later in southern China, that phytoplasmas are also associated with HLB symptoms. The photo shows two leaves with characteristic HLB blotchy mottle symptoms associated with the 16Sr group IX phytoplasma (São Paulo State, Brazil) - D. C. Teixeira and J. M. Bové |
||
|
Additional sweet orange leaves with characteristic HLB blotchy mottle symptoms associated with the 16Sr group IX phytoplasma (São Paulo State, Brazil) - D. C. Teixeira and J. M. Bové |
||
|
The symptoms associated with the 16Sr group IX phytoplasma are not only leaf symptoms, but also fruit symptoms, as shown on this photo: lopsideness, colour inversion, brownish stained vascular bundles, aborted seeds (São Paulo State, Brazil) - D. C. Teixeira and J. M. Bové |
||
|
Crotolaria juncae plants, grow in-between citrus rows for soil improvement, are also found to be infected with the 16Sr group IX phytoplasma, and show typical symptoms of witches’ brooms, as illustrated on the photo (São Paulo State, Brazil) - N. A. Wulff and J. M. Bové |
||
|
The three HLB-associated Candidatus (Ca.) Liberibacter (L.) species could be transmitted by dodder (Cuscuta campestris) from graft-inoculated sweet orange seedlings to periwinkle (Catharanthus roseus) plants: Ca. L. africanus and Ca. L. asiaticus in 1982, and Ca. L. americanus in 2005. The liberibacter titer in periwinkle plants is higher than in citrus (Bordeaux, France) - M. Garnier, P. Bonnet, and J. M. Bové |
||
|
Ca. L. asiaticus (Philippines strain) was also dodder-transmitted to tobacco (Nicotiana tabacum xanthi) plants, in which it induced severe symptoms, as shown on the photo (Bordeaux, France) - M. Garnier and J. M. Bové |
||
|
Electron micrograph showing liberibacter cells (arrows) in tobacco sieve tubes. Titer of liberibacters in tobacco is lower than in periwinkle plants (Bordeaux, France) - M. Garnier and J. M. Bové |
||
|
Fruiting Murraya paniculata tree. This citrus relative is the preferred host of Diaphorina citri, the psyllid vector of Ca. L. asiaticus and Ca. L. americanus in São Paulo State, Brazil. Control of HLB in São Paulo State implies that all M. paniculata trees be eradicated and that production of new Murraya trees be prohibited (São Paulo State, Brazil) – J. M. Bové |
||
|
In the Cape province of South Africa, “Cape chesnut” (Calodendron capense) trees (1 and 3), showed blotchy mottle leaves (2), and were found to be infected with a liberibacter characterized as subspecies “capensis” of the African liberibacter, Ca. L. africanus (Cape province, South Africa) – M. Garnier, H. Le Roux, and J. M. Bové |
||
|
The two psyllid vectors of HLB – D. Catling |
||
|
Trioza erytreae, the African citrus psyllid, which transmits Ca. L. africanus in Africa, Madagascar, Reunion and Mauritius islands, Yemen,…Experimentally, it also transmits Ca. L. asiaticus. |
||
|
Upper left: yellow eggs of Trioza erytreae are seen along the midrib and on the edges of a young lemon leaf. Nymphs of T. erytreae develop in individual pits or nests on the lower leaf surface, and to each nest corresponds, on the upper leaf surface, a “bump”. The three leaves of A show, on their lower leaf surface, the nests left empty by the development of the nymphs into adults, and the upper surface of the three same leaves (B) shows the bumps corresponding to the nests. Some citrus relatives such as Toddalia lanceolata (C) are also hosts of T. erytreae, as shown by the “bumps” – J. M. Bové |
||
|
Diaphorina citri, the Asian citrus psylid, transmits Ca. L. asiaticus in Asia and America, as well as Ca. L. americanus in Brazil. Experimentally, it also transmits Ca. L. Africanus. Upper right: D. citri feeding on citrus shoot in reunion island (Courtesy B. Aubert). Lower right: D. citri feeding on sweet orange leaf in Behai, Guangxi, China. Lower left: D. citri feeding on sour orange leaf in Peshawar, Pakistan. Notice position of insect at 45° angle with its support. – J. M. Bové |
||
|
Diaphorina citri nymphs and adults on lime (Citrus aurantifolia) shoots. Notice heavily curled leaves and production of honey dew by the insects. D. citri is not only a vector, but also a pest (Saudi Arabia) – J. M. Bové |
||
|
Graft transmission of Ca. L. americanus by graft-inoculation (Bordeaux, France) - J. M. Bové and P. Bonnet |
||
|
Seasonal symptom expression of HLB in São Paulo citrus groves. Expression of HLB symptoms is much better in the cool, autumn and winter months (March to September). Therefore the number of symptomatic trees detected in that period is much higher than in the spring and summer months (São Paulo State, Brazil) - R. B. Bassanezi |
||
|
Spatial distribution of HLB-symptomatic trees in a citrus grove in São Paulo State, Brazil. The photo illustrates the “edge effect”, namely the fact that symptomatic trees are concentrated at the borders of groves. This edge effect is well seen on this photo, as the symptomatic trees, once identified, have been removed - R. B. Bassanezi |
||
|
Spatial distribution of HLB-symptomatic trees in a citrus grove in São Paulo State, Brazil. The photo illustrates the “core clusters” and the “reflected clusters” - R. B. Bassanezi |
||
|
Summary of the major leaf and fruit symptoms of HLB: leaf blotchy mottle, fruit colour inversion, lopsidedness, aborted seeds, and brownish-stained vascular bundles in fruit axis at peduncular end – J. M. Bové |
||
|
Comparison of fruit symptoms of stubborn with those of HLB. Acorn-shaped fruits are characteristic of “stubborn”, a disease caused by the helical mycoplasma, Spiroplasma citri. The two acorn-shaped navel sweet oranges in the bottom part of the photo have a coarse rind at the peduncular ends of the fruits (reminiscent of the acorn cupule), and a smooth rind at the stylar ends (reminiscent of the smooth acorn part, which sticks out of the cupule). The two half fruits at the upper right represent sections through acorn-shaped fruits, and show that the albedo at the peduncular end is thick, while at the stylar end, it is very thin. Also, the navels of acorn-shaped fruits are often more or less closed. Stubborn fruits can also be lopsided (upper left) and resemble HLB-affected fruit. See also (HLB. 122) – Juan Gil |
||
|
Comparison of fruit symptoms of stubborn with those of HLB. Both stubborn-affected sweet oranges (lower part of photo) and HLB-affected sweet oranges (upper part of photo) show colour inversion. Stubborn-affected fruits, like HLB-affected fruits, may be lopsided. Other fruit symptoms shared by the two diseases include seed abortion, brownish-stained vascular bundles at the peduncular end of the fruit axis, thick albedo at the peduncular fruit end, thin albedo at the stylar end. However, the albedo at the stylar end is thinner with stubborn than with HLB (São Paulo State,Brazil, and Erzin, Turkey) – J. M. Bové |
||
|
Comparison of leaf symptoms of HLB with those of citrus variegated chlorosis (CVC). In addition to leaf blotchy mottle, HLB-affected trees often show leaves with zinc deficiency symptoms. These symptoms should not be confused with CVC leaf symptoms (A) which have yellow discolorations in the interveinal tissue, and resemble those of zinc deficiency. Confusion can be avoided by examining the lower leaf suface; in the case of CVC, brownish-stained material is present in the areas of yellow discoloration, leading sometimes to tissue necrosis. No such material is present in the case of genuine zinc deficiency or HLB-induced zinc deficiency (São Paulo State, Brazil) – J. M. Bové |
||
|
Electron microscopy detection of the HLB liberibacter in citrus from several Asian countries (Bordeaux, France) – M. Garnier and J. M. Bové |
||
|
Electron microscopy detection of the HLB liberibacter in citrus from several African countries (Bordeaux, France) – M. Garnier and J. M. Bové |
||
|
Conditions for detection and identification of the HLB liberibacters in citrus by electron microscopy (Bordeaux, France) – M. Garnier and J. M. Bové |
||
|
Detection and identification of the HLB phytoplasmas in citrus by electron microscopy. Phytoplasmas have been found associated with HLB symptoms in Brazil and China. Like the liberibacters, they are restricted to the sieve tubes, but contrary to the liberibacters, they have no cell wall, their cytoplasmic membrane being their only cell envelope (See also HLB. 101). (São Paulo State, Brazil) – E. W. Kitajima and J. M. Bové |
||
|
Detection of HLB liberibacters by immunifluorescence with liberibacter-specific monoclonal antibodies (Bordeaux, France) – M. Garnier, S. Gao and J. M. Bové |
||
|
Cells of Ca. Liberibacter asiaticus purified by immunoaffinity chromatography and labelled with colloidal gold (Bordeaux, France) – M. Garnier, S. Villechanoux and J. M. Bové |
||
|
Multiplex PCR detection of Ca. L. americanus, Ca. L. asiaticus, and Ca. L. africanus with two sets of primers: description of primers (São Paulo State, Brazil) - D. C. Teixeira and J. M. Bové |
||
|
Multiplex PCR detection of Ca. L. americanus, Ca. L. asiaticus, and Ca. L. africanus with two sets of primers: visualization of amplicons by agarose gel electrophoresis (São Paulo State, Brazil) - D. C. Teixeira and J. M. Bové |
||
|
Sensitivities of various PCR methods for detection of Ca. L. americanus. As shown on the table, 16S rDNA PCR still gives a positive reaction (+) with sample 3, which corresponds to 104 liberibacters/assay (l/a); rpl PCR is 10 times more sensitive, as it gives a positive reaction with sample 4 (103 l/a); nested PCR and Real Time PCR are 1000 times more sensitive as they give positive reactions with sample 6 (10 l/a) (Bordeaux, Fance) - D. C. Teixeira, C. Saillard, S. Eveillard and J. M. Bové |
||
|
“Closed” nursery for the production of nursery trees free of insect-transmitted diseases (São Paulo State, Brazil) – A. J. Ayres |
||
|
Rear view of tractor and “platform” with two inspectors to survey young orchards for identification of trees with HLB symptoms (São Paulo State, Brazil) – F. Tersi |
||
|
Rear view of tractor and two-inspector-platform to survey orchards of middle-size trees for HLB symptoms (São Paulo State, Brazil) - J. M. Bové |
||
|
Front view of tractor and platform of photo (HLB. 135) (São Paulo State, Brazil) - J. M. Bové |
||
|
Four-inspector platform to survey large tree orchards for HLB symptoms. This platform allows simultaneous inspection of the sides of the trees and of the tree tops, where symptoms often show up (São Paulo State, Brazil) - J. M. Bové |
||
|
Two-inspector platform for inspection of tops of large trees. See also (HLB. 137) (São Paulo State, Brazil) - J. M. Bové |
||
|
Once identified, symptomatic trees must be removed. This can be done by cutting the trees with a saw (A), with a clipper (B), or by pulling the tree out of the soil (C). With method A and B, the tree stumps remain in the soil; with method C, roots remain in the soil. The development of shoots (D) on the remaining stumps and/or roots must be prevented (São Paulo State, Brazil) - J. M. Bové |
||
|
Application of insecticide on tree trunks with the “Calibra” machine (South Africa) – H. Le Roux |
||
|
Control of HLB. Example of a citrus farm with good HLB control (São Paulo State, Brazil) – J. Belasque |
||
|
Control of HLB. Another example of a citrus farm with good HLB control (São Paulo State, Brazil) – J. Belasque |
||
|
Control of HLB. Example of a citrus farm where control might have been better with more treatments against the psyllid vectors (São Paulo State, Brazil) – J. Belasque |
||
|
Control of HLB. Example of a farm, in which the HLB control system has not given satisfactory results because of several neighbouring farms where no HLB control has been carried out. (São Paulo State, Brazil) – J. Belasque |
||
|
Biological control of the psyllid vectors of HLB, Diaphorina citri and Trioza erytreae, in Reunion island with Tamarixia radiata and Tamarixia dryi, respectively (Reunion island, France) – J. Etienne and B. Aubert |
||
|
Nymphs of Trioza erytreae parasitized by the ectoparasite, Tamarixia dryi – B. Aubert, E. C. G. Bedford and J. M. Bové |