Concave Gum - Blind Pocket
HISTORY, DISTRIBUTION AND IMPORTANCE
History. The history of concave gum - blind pocket is complicated, if not confusing, as it is mixed with that of several other citrus diseases: psorosis, crinkly leaf, infectious variegation, cristacortis and impietratura.
The name “psorosis” was selected by Swingle and Webber (1896) to describe a disease characterized by bark scaling lesions on trees of sweet orange and other varieties (CG-BP. 01). In addition to bark scaling, the disease was found by Fawcett (1933) to have also leaf symptoms, the so-called psorosis young-leaf flecking symptoms (CG-BP. 02). Two forms of psorosis were distinguished: “psorosis A”, the common form of the disease, and “psorosis B”, a less common but more severe form, characterized by early bark lesions, mature-leaf symptoms (CG-BP. 02), and twig dieback (Fawcett, 1932; Fawcett and Klotz, 1938). Next, it was shown by Fawcett and Cochran (1942): (i) that psorosis B could be produced by inoculating sweet orange seedlings with bark patches taken into a lesion of psorosis A (so-called “lesion bark”), and (ii) that psorisis B was probably not caused by an agent different from that of psorosis A. Finally, Wallace (1957) demonstrated that sweet orange seedlings did not develop psorosis B upon inoculation with psorosis A lesion bark if they had been inoculated previously with Psorosis A non-lesion bark. In other words, inoculating a sweet orange seedling first with Psorosis A non-lesion bark, and next challenging them with lesion bark (challenge inoculation), will “cross-protect” the sweet orange seedling against the development of psorosis B symptoms. This cross-protection reaction became a powerful test to identify psorosis A and distinguish it from other disorders, which may show various leaf symptoms that could be mistaken for psorosis.
Fawcett was also involved in the first reports on concave gum (CG-BP. 03), blind pocket (CG-BP. 04) and crinkly leaf (CG-BP. 05). These diseases were first mentioned in 1926 (Fawcett and Lee, 1926), and further information was provided by Fawcett ten years later (Fawcett, 1936). He found that these three diseases had young-leaf symptoms very similar to the psorosis flecking symptoms, the ones he had reported in 1933 (Fawcett, 1933). Young leaves of Concave gum-affected trees not only had the psorosis young-leaf flecking, but also zonate or oak leaf pattern (CG-BP. 06).
Thus, psorosis young-leaf symptoms represented a common characteristic of psorosis A, concave gum, blind pocket and crinkly leaf. In addition, these symptoms could be transmitted by graft-inoculation. For these two reasons, concave gum, blind pocket and crinkly leaf were thought to be members of the “psorosis family” of diseases (Fawcett, 1939), all four diseases being caused by related strains of the same putative “virus” (viruses were the only type of infectious agents known in those days). Finally, in 1939, Fawcett and Klotz described a “psorosis-like” disorder of lemon, infectious variegation (CG-BP. 05), and suggested its relationship to crinkly leaf (Fawcett and Klotz, 1939), and so infectious variegation was added to the psorosis family of diseases. Descriptions of the various psorosis types and apparent evidence of their relationships were published in the years to follow (Klotz and Fawcett, 1941; Fawcett and Bitancourt, 1943). Even at the first International conference on citrus virus diseases in 1957 in Riverside, California, concave gum, blind pocket, crinkly leaf, and infectious variegation were still considered part of the psorosis group (Wallace, 1959).
Lilian Fraser in Australia was the first to challenge inclusion of crinkly leaf in the psorosis virus group (Fraser, 1961). No psorosis A young-leaf symptoms were produced by any of the many seedlings inoculated with bark from crinkly leaf-affected trees, provided that they were free of psorosis A. She suggested that the experiments in California, which led to linking crinkly leaf with psorosis, were conducted with citrus material carrying both psorosis A and crinkly leaf. Also, in early studies it was found that crinkly leaf and infectious variegation cross-protected sweet orange against psorosis A lesion inoculum. However, later work showed that cross-protection was also due to contamination of the crinkly leaf and infectious variegation sources with a psorosis A virus. In the case of crinkly leaf, the contaminating psorosis virus could be eliminated through seed- or mechanical-transmission, and the remaining crinkly leaf virus was found unable to induce the cross-protection reaction (Wallace, 1968). Similar results were also obtained with uncontaminated infectious variegation sources cleaned by mechanical transmission. Finally, Dauthy and Bové (1964, 1965, and 1968) purified crinkly leaf virus and infectious variegation virus and concluded that they were strains of the same virus. Similar conclusions were obtained by Majorana and Martelli (1968). It is known today that the agents of crinkly leaf, infectious variegation as well as leaf rugose are members of the ILAR virus group (Rana et al., 1974; Gonsalves and Garnsey, 1976). They are unrelated to the psorosis virus.
The first suggestion that concave gum was unrelated to psorosis came from cross-protection experiments by Roistacher and Calavan (1965) who showed that a number of concave gum sources did not protect sweet orange seedlings against psarosis A lesion bark. Similar results were eventually obtained by Wallace (see Wallace, 1978). Cross-protection experiments with blind pocket sources gave rather confusing results. However, concave gum and blind pocket being diseases with very similar symptoms and characteristics, they were both removed from the psorosis group.
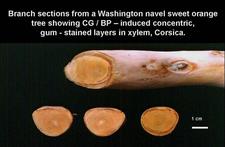
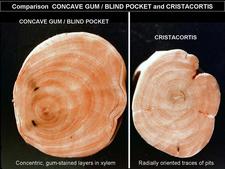
Vogel and Bové (1964, 1968, 1972), when studying cristacortis (CG-BP. 07), always observed psorosis young-leaf symptoms, not only flecking but also oak-leaf patterns (CG-BP. 08), on citrus plants infected either naturally or experimentally (by graft-inoculation) with the cristacortis pathogen. As oak-leaf patterns were thought to be characteristic of concave gum, and since many cristacortis sources showed or induced such leaf symptoms in inoculated navel sweet orange or Orlando tangelo seedlings, it was concluded, initially, that the young-leaf symptoms were caused by the concave gum agent, co-infecting the cristacortis sources (Vogel and Bové, 1972). However, sources of cristacortis free of concave gum could be identified on the basis of internal wood symptoms (Vogel and Bové, 1974). Indeed, the Concave gum agent induces gum deposits in certain annual growth rings in the wood. As the growth rings are concentric, the gum-stained layers have also a concentric appearance (CG-BP. 08). On the contrary, cristacortis induces radially oriented stem pits (CG-BP. 08). Furthermore, traces of previous, radially oriented pits remain buried in the wood (CG-BP. 29). Sources carrying both concave gum and cristacortis induce the concentric gum-stained layers as well as the radially oriented traces. On the basis of these criteria, sources of cristacortis free of the concave gum agent were identified, and these sources showed and induced young-leaf symptoms including oak-leaf patterns on inoculated citrus seedlings. These results showed that concave gum and cristacortis were different diseases, but both were associated with the same young-leaf symptoms (CG-BP. 06) (Vogel and Bové, 1974).
Impietratura was first described as “Samrah” by Reichert and Hellinger (1930) in Palestine. The disease, under the name of “impietratura”, has long been known to Sicilian citrus growers, and is widely spread among Mediterranean countries (Ruggieri, 1955). Affected fruits are as hard as “stone”, hence the name “impietratura”, and contain pockets of gum in the albedo (CG-BP. 09). Ruggieri (1961) demonstrated that impietratura was transmissible by graft inoculation. Bar-Joseph and Loebenstein (1970) showed that budwood from infected trees induced flecking and oak-leaf pattern on citrus indicator seedlings, and these leaf symptoms could be used for indexing. However, in the absence of “pur” impietratura sources, the possibility could not be ruled out that the leaf symptoms were induced by some other agent, such as the concave gum agent, putatively present in all the impietratura trees examined.
Recent data have confirmed that there are no more reasons to include impietratura into a psorosis group, than there are to include concave gum-blind pocket and cristacortis. Indeed, the virus causing psorosis A has been identified and characterized in the 1990s as a bipartite, single stranded RNA ophiovirus (CpsV) (Martin et al., 2005) In particular, a 48kDa protein, probably the coat protein, is characteristic of CpsV-infected citrus. The 48kDa protein is absent from citrus infected not only with concave gum-blind pocket, but also cristacortis and impietratura, and thus these diseases are unrelated to psorosis, even though they are associated with “psorosis” young-leaf flecking symptoms (da Graça et al., 1991). At this moment, the agents responsible for the ”Oak-leaf pattern diseases” (concave gum-blind pocket, cristacortis, impietratura) have not been identified.
Distribution and importance. The disease is largely present in the Mediterranean region although it is now found worldwide. Injury develops slowly but may distort trunks and branches considerably (CG-BP. 03, 10, 11, 12, 13) and damage trees severely. Many spectacular cases were seen on old line mandarin and sweet orange trees in Corsica (Vogel, 1961). Concave gum disease can be quite severe and very debilitating to young trees (D'Onghia et al., 1992). As the disease is not transmitted by insects or mechanically, it has disappeared from orchards started with citrus material certified free of graft-transmissible agents.
NAME AND SYNONYMS OF THE DISEASE
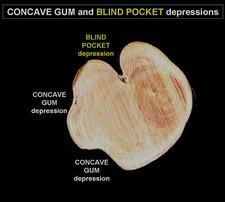
Concave gum: named from (i) the broad concavities or depressions of various sizes on the trunk and limbs (CG-BP. 03, 10, 11, 12, 13), (ii) the presence of gum beneath the concavities, in-between wood and bark (CG-BP. 14), and (iii) gum exudation through bark-cracks in the center or around the margins of the depression at certain seasons (CG-BP. 15). The disease was formerly called concave gum “psorosis”. It is however not related to psorosis (see history).
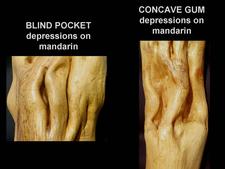
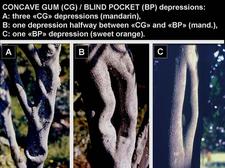
Blind pocket: named from pocket-like depressions, deeper and narrower than the concave gum depressions (CG-BP. 04, 16. 17) While concave gum depressions are “open”, with opposite margins relatively far apart, blind pocket depressions are “closed, with opposite margins almost touching each other (CG-BP. 18, 19). However, it is not always easy to distinguish between the two types of depressions, and depressions half way between concave gum depressions and blind pocket depressions do occur (CG-BP. 20). The agents of concave gum and blind pocket are graft-transmissible but have not yet been identified. On the basis of similar trunk and leaf symptoms (flecking and oak-leaf pattern) they are thought to be closely related and are considered here as one single disease: concave gum-blind pocket, one of the members of the ”oak-leaf pattern diseases”.
Abbreviation: CG‑BP for Concave Gum ‑ Blind Pocket.
SYMPTOMATOLOGY
General aspect of affected field trees
Mild to severe stunting. Trunk and large limbs are gnarled and distorted, (making them most suitable for elegant lamp-stands of valuable citrus-wood). In Mediterranean countries where sour orange is still the major rootstock, trunk symptoms are characteristically only seen on the sensitive scion: mandarin (CG-BP. 03, 11), Clementine (CG-BP. 10), sweet orange, grapefruit, tangelo (CG-BP. 12, 13) but not on the tolerant sour orange stock (CG-BP. 12, 13). The same situation may occur with other tolerant rootstocks (lemon, P. trifoliata). Concavities developing on the scion trunk close to the bud union line may distort the sour orange tissues immediately below the bud union (CG-BP. 19).
Symptoms on trunk (on rootstock and/or scion), limbs and shoots
Concave gum: (i) Conspicuous broad concavities or depressions, 10-20 cm or more in length and half as wide (CG-BP. 03, 10, 19, 20), (ii) presence of gum between bark and wood at depressions (CG-BP. -/14), (iii) gum oozing through cracks in the bark (CG-BP. 15), (iv) gummy, granulated tissue under the bark, cinnamon or russet in color, and (v) as seen on cross sections of small (CG-BP. 21) or large (CG-BP. 22) branches or limbs, some of the concentric growth rings in the wood may be stained with brownish gum-like material especially underneath depressions (CG-BP. 21, 22). The distorted, gnarled aspect of trunks and limbs affected by CG-BP is due to the development of numerous, partly overlapping depressions.
Blind pocket : depressions are deeper, narrower, and more numerous than those of concave gum. (CG-BP. 04, 16, 17, 18, 19, 20).
Often concave gum and blind pocket depressions occur together on the same tree (CG-BP. 11, 12, 18)
Symptoms on leaves
Trees affected by CG-BP show (i) young-leaf flecking symptoms (CG-BP. 23), also seen on psorosis A-affected trees or inoculated seedlings, and (ii) young-leaves with oak leaf patterns (CG-BP. 23, 24). These young leaf symptoms are more generally observed in the spring and fall flushes of growth, when outside temperatures are still relatively mild. In the spring, the oak leaf pattern can be seen throughout the tree on almost all of the leaves in the young flush of growth. Symptoms on the young leaves gradually disappear as the leaves mature. Symptoms are seen most readily when the leaf is shaded (with one hand) from the direct sun and viewed against the light of the sky.
Symptoms on fruit
None
Histological symptoms
At the sites where depressions occur, wood development seems to be inhibited (Wallace, 1959, 1978). In the case of BP depressions, a smaller area of wood is affected, so that when growth continues on each side of the affected wood, the depression becomes very narrow. In the case of long-existing blind pocket depressions, the points of origin are deep within the wood (CG-BP. 18). At the point of origin, the cambial side of the invaginated bark has no peg formations fitting the narrow wood pocket. In the case of cristacortis, such pegs do occur and are characteristic.
CAUSAL AGENT: DESCRIPTION AND PROPERTIES
The causal agent is readily transmitted by graft-inoculation. Its nature is unknown.
HOST RANGE
Thus far restricted to citrus spp. Non-rutaceous hosts are not known.
Sensitive species, varieties or combinations
Sweet orange (CG-BP. 04), mandarin (CG-BP. 03, 11), clementine (CG-BP 10), tangelo (CG-BP. 12, 13), grapefruit show the depressions characteristic of CG-BP. In Japan, severe symptoms of the disease occur on pumelo-like hyuganatsu (Citrus tamurana) (Ieki and Ito, 1996).
During their early stages of life, infected trees may not show the depression symptoms. However, they may show the oak-leaf patterns common to CG-BP, cristacortis, and impietratura.
Symptomless species, varieties or combinations
Sour orange (CG-BP. 03, 12, 13), trifoliate orange and lemon do not show trunk and branch depressions and are considered symptomless carriers for these symptoms.
Sour orange, lemon, citron may show oak-leaf patterns, but no trunk or branch symptoms.
TRANSMISSION
Natural
Transmission is primarily by man through infected budwood. There is no evidence of vector transmission.
Experimental
The agent is readily transmitted by graft-inoculation of bark patches, buds or shoots. A high percentage of transmission is obtained.
Mechanical transmission: none reported.
Transmission of young-leaf symptoms was obtained in Corsica by placing pollen from a Parson Brown sweet orange tree infected with the agents of Concave gum (California strain 158-62) and exocortis under the bark of six tangelo seedlings. All six seedlings showed young-leaf symptoms ((flecking and oak-leaf pattern) within five years after pollen treatment. However, trunk or limb symptoms (concavities or depressions) had not appeared after eight years (Vogel and Bove, 1980). In the case of cristacortis, young-leaf symptoms (flecking and oak-leaf pattern) as well as stem pitting were obtained in five of six Orlando tangelo seedlings treated with pollen from an Orlando tangelo tree infected with cristacortis, concave gum and exocortis (Vogel and Bove, 1980). However no natural transmission of young-leaf symptoms and/or cristacortis symptoms has ever been observed in Corsica.
EPIDEMIOLOGY
Not studied
DIAGNOSIS
Diagnostic field symptoms
Broad concave depressions, narrow pocket-like depressions, intermediate depressions on trunk, main limbs, and branches (CG-BP. 19, 20). With tolerant rootstocks such as sour orange in many Mediterranean countries, depressions and distortions will only affect the scion trunk, not the sour orange rootstock. Photos CG-BP. 03, 04, 10, 11, 12, 13 show trunks highly characteristic of concave gum-blind pocket.
After having identified the depressions, look for young-leaf symptoms with oak-leaf pattern on spring and fall flushes of growth. For confirmation or in case of doubt, index for oak-leaf pattern (see below: biological indexing, leaf symptoms).
Field trees with oak-leaf pattern on young leaves, but without trunk, limb or branch symptoms, may be affected with any one of the three “oak-leaf pattern” diseases (CG-BP, cristacortis, and impietratura). Indexing on seedlings of sour orange, navel sweet orange, Orlando tangelo, and fruit-bearing grapefruit tree (CG-BP. 09) will give the results shown on the following table, and make it possible to identify the agent(s) involved. This indexing might take several years. In many cases, identification of the agent(s) is not required, as leaf symptoms alone are sufficient to indicate that the candidate tree is infected.
|
|
Trunk or branch symptoms on |
Fruit symptoms on |
||
|
Sour orange seedling |
Navel sweet orange seedling |
Orlando tangelo seedling |
Fruit-bearing grapefruit tree ** |
|
|
CG-BP |
-* |
Depressions, Gumming |
Depressions, gumming |
|
|
Cristacortis |
Stem pitting |
Stem pitting |
Stem pitting |
|
|
Impietratura** |
- |
- |
- |
Fruit with gum in albedo |
* - : no symptoms.
** Fruit-bearing grapefruit trees are required only for impietratura indexing (CG-BP. 09).
Comparison with other diseases
Leaf symptoms
Young leaf symptoms of Psorosis-A are only of the “flecking” type. Young leaf symptoms of CG-BP, but also of cristacortis and impietratura, are of two types: flecking and the characteristic oak-leaf pattern (CG-BP. 06). However, the first symptoms to appear on the young emerging flush of seedlings of sweet orange, mandarin, or Dweet tangor, within 5 to 8 weeks after inoculation, is interveinal flecking (CG-BP. 39), and oak leaf patterns are usually not yet seen in this initial symptom. In the absence of branch or fruit symptoms, presence of oak-leaf pattern indicates infection with CG-BP, cristacortis or impietratura.
Symptoms on trunk and branches
Symptoms of cristacortis resemble those of CG-BP, but the depressions due to cristacortis (CG-BP. 07, 25, 27) are smaller than the concavities or pockets of CG-BP. With cristacortis, there are pegs on the inner side of the bark that go deep into corresponding pits in the wood (CG-BP. 07, 26, 27). No such pegs and pits develop with CG-BP. With CG-BP, gum deposits are seen in some of the concentric growth layers of the xylem (CG-BP. 21, 22, 28), while with cristacortis, radially oriented traces of old pits remain buried in the xylem (CG-BP. 28, 29, 30).
Stem pitting symptoms of cristacortis are frequently seen on sour orange rootstocks (CG-BP. 12, 25); symptoms of CG-BP are never seen on sour orange rootstocks (CG-BP. 03, 11, 13, 16).
On varieties such as mandarin or tangelo, susceptible to cachexia, cristacortis and CG-BP, stem pitting symptoms of cristacortis (CG-BP. 31) or cachexia (CG-BP. 32) can be overimposed on the CG-BP depressions.
Concavities of Tarocco pit (Russo and Klotz, 1963), unlike those of concave gum, are often round, more numerous and exude a ground, corklike material (CG-BP. 33).
Deep pits associated with corresponding pegs on the cambial side of bark were observed on Rubidoux trifoliate orange rootstock infected with citrus bent leaf viroid (CBLVd) variant-1a (Roistacher et al, 1993). Similar symptoms were also seen in Corsica on Pomeroy trifoliate orange rootstocks in an extensive viroid assay involving various variants of CEVd, HSVd, CDVd, CBCVd, and variant 1a and 1b of CBLVd (Vernière et al., 2004). However, these symptoms were not the result of viroid infections as they were present in trees of all treatments, including the non-inoculated controls. Furthermore, the pits resemble more cristacortis pits than CG-BP depressions, since they have corresponding pegs on the cambial side of the bark.
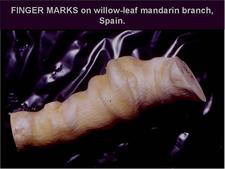
Fingers marks (CG-BP. 34) have sometimes been associated with CG-BP but they are not graft-transmissible.
Biological indexing
Indicator citrus seedlings
For oak-leaf patterns: mandarin, Dweet tangor or sweet orange seedlings, but citron and lemon will also show oak-leaf patterns.
For trunk and branch symptoms: Washington navel sweet orange and Orlando tangelo.
Symptoms
Sweet orange seedlings graft-inoculated with non-lesion bark from Psorosis-A-infected trees show “shock symptoms” (wilting, bending and decline of the first young shoots developing after inoculation and cutting back the index plant) (CG-BP. 40). Shock symptoms do not occur after inoculation of bark from CG-BP infected trees.
Leaf symptoms. Very mild interveinal streaking or flecking is the very first symptom after graft-inoculation of the indicator seedlings (CG-BP. 39). Usually, oak-leaf patterns are best observed on the second flush of growth (CG-BP. 35, 36, 37); they appear in the expanding leaves, and are best before the expanded leaves harden; they disappear completely as the leaves mature and harden. If temperatures during indexing are too high, symptoms in sweet orange or Dweet tangor indicator seedlings are completely masked (CG-BP. 36). The recommended greenhouse temperatures are 24-27° C during the day and 18-21°C at night (Roistacher, 1991). Cool night conditions, especially in the spring and fall flush of growth, favor the development of oak leaf patterns.
Wood symptoms. On inoculated seedlings of Washington navel sweet orange and Orlando tangelo, CG-BP depressions (CG-BP. 38) develop earlier than on other citrus species, and characteristic wood symptoms of CG-BP [concentric gum-stained growth layers in the xylem (CG-BP. 21)and gum oozing (CG-BP. 14)] often develop within two to three years, even before CG-BP depressions on the trunk and branches can be seen. The gum-stained layers frequently appear in shoots with very small diameters. On Orlando tangelo seedlings, the typical CG-BP depressions develop within six years, while cristacortis stem pitting is obtained within 3 years (Vogel and Bové, 1974).
CONTROL
Use budwood free of CG-BP. The agents associated with CG-BP and the other two oak-leaf pattern diseases, cristacortis and impietratura, are readily eliminated by shoot tip grafting (STG). After STG, indexing must confirm absence of the CG-BP agent in the shoot tip-borne material: leaves of indicator seedlings inoculated with shoot tip-borne material must remain free of oak-leaf patterns.
Thermotherapy (Calavan et al., 1972; Roistacher, 1977) can also be used to eliminate the agent of CG-BP.
SELECTED REFERENCES
Bar-Joseph, M., G. Loebenstein (1970). Leaf flecking on indicator seedlings with citrus in Israel. A possible indexing method. Plant Dis. Reptr. 54: 643-646.
Calavan, E.C., C.N. Roistacher, E.M. N auer (1972). Thermotherapy of citrus for inactivation of certain viruses. Plant Dis. Rep. 56:976-980.
da Graça, J. V., R. F. Lee, P. Moreno, E. L. Civerolo, K. S. Derrick (1991). Comparison of citrus ringspot, psorosis, and other virus-like agents of citrus. Plant Dis. Reptr. 75: 613-616.
Dauthy, D., J. M. Bové (1964). Isolement du virus de la frisolée des citrus. Note préliminaire. Fruits 19 : 187-193.
Dauthy, D., J. M. Bové (1965). Experiments on mechanical transmission of citrus viruses. p. 250-253. In W. C. Price (ed.) Proc.3rd Conf. Intern. Organization Citrus Virol., Univ. Florida Press, Gainesville.
Dauthy, D., J. M. Bové (1968). Purification of crinkly-leaf virus. p. 255-263. In J. F. L. Childs (ed.) Proc.4th Conf. Intern. Organization Citrus Virol., Univ. Florida Press, Gainesville..
D'Onghia A.M., P. de Marco and V. Savino (1992) - Gravi casi di concavita' gommose su navalina in Puglia. (Severe cases of concave gum on Navalina orange in Apulia. Informaatore Fitopatologico 3:39-41.
Fawcett, H. S. (1932). New angles on treatment of bark diseases of citrus. California Citograph 17: 406-408.
Fawcett, H. S. (1933). New symptoms of psorosis, indicating a virus disease of citrus. (Abstr.) Phytopathology 23: 930.
Fawcett. H.S. (1936). Citrus diseases and their control. 656 pp. McGraw Hill Book Co. Inc. New York, London.
Fawcett. H.S. (1939). Psorosis in relation to other virus-like effects on citrus. (Abstr.) Phytopathology 29: 6.
Fawcett H.S. and A.A. Bitancourt (1943). Comparative symptomatology of psorosis varieties on citrus in California. Phytopathology 33: 837-864.
Fawcett, H. S., L. C. Cochran. (1942). Symptom expression in psorosis of citrus as related to kind of inoculum. (Abstr.) Phytopathology 32: 22.
Fawcet, H. S., L. J. Klotz. (1938). Types of symptoms of psorosis and psorosis-like diseases of citrus. (Abstr.) Phytopathology 28: 670.
Fawcet, H. S., L. J. Klotz. (1939). Infectious variegation of citrus. Phytopathology 29: 911-912.
Fawcett, H. S., H. A. Lee. (1926). Citrus diseases and their control. McGraw-Hill, New York and London. 582 pp.
Fraser, L. (1961). Lemon crinkly leaf virus. p. 205-210. In W. C. Price (ed.) Proc. 2nd Conf. Intern. Organization Citrus Virol., Univ. Florida Press, Gainesville.
Gonsalves, D., S. M. Garnsey. (1976). Association of particle size with sedimentation velocity of the nucleoprotein components of citrus variegation and citrus leaf rugose viruses. p. 109-115. In E. C. Calavan (ed.) Proc. 7th Conf. Intern. Organization Citrus Virol., IOCV, Univ. Calif., Riverside.
leki, H and T. Ito. (1996). Occurrence of concave gum on Hyuganatsu (Citrus tamitrana) in Japan, p.346-348. In J. V. da Graça, P. Moreno, R. K. Yokomi (eds)Proc. 13th Conf. Intern. Organization Citrus Virol., IOCV, Univ. Calif., Riverside.
Klotz, L. J., H. S. Fawcett. (1941). Color handbook of citrus diseases. Univ. Calif. Press, Berkeley and Los Angeles. 90 pp.
Majorana, G., G. P. Martelli. (1968). Comparison of citrus infectious variegation and citrus crinkly leaf virus isolates from Italy and California. p. 273-280. In J. F. L. Childs (ed.) Proc.4th Conf. Intern. Organization Citrus Virol., Univ. Florida Press, Gainesville.
Martin, S., C. Lopez, M. L. Garcia, G. Naum-Ongania, O. Grau, R. Flores, P. Moreno, J. Guerri. (2005). The complete nucleotide sequence of a Spanish isolate of citrus psorosis virus: comparative analysis with other ophioviruses. Archives of Virology 150: 167-176.
Rana, G. L. , A. Quacquarelli, G. P. Martelli, (1974). Some properties of citrus variegation virus. p. 165-168. In L. G. Weathers, M. Cohen (eds) Proc. 6th Conf. Intern. Organization Citrus Virol., Univ. Cali., Div. Agr. Sci.
Reichert, I., E. Hellinger. (1930). Internal decline. A new physiological disease of citrus fruits in Palestine. Hadar 3: 220-224.
Roistacher, C.N.) (1977). Elimination of citrus pathogens in propagative budwood. I. Budwood selection, indexing and thermotherapy. Proc. Int. Soc. Citriculture 3: 965-972.
Roistacher, C.N.) (1991). Graft-transmissible diseases of citrus. Handbook for detection and diagnosis. 286 pgs. Publication Division Food and Agricultural Organization of the United Nations, Viale delle Terme di Caracalla, 00100 Rome, Italy.
Roistacher C.N., E.C. Calavan (1965). Cross protection studies with strains of concave gum and psorosis viruses. p. 154-161. In W.C. Price (ed.) Proc. 3rd Conf. Intern. Organization Citrus Virol., Univ. Florida Press, Gainesville.
Roistacher, C. N., J. A. Bash and J. S. Semancik. (1993). Distinct disease symptoms in Poncirus trifoliata induced by three citrus viroids from three specific groups, p. 173-179. InProc. 12th Conf. Intern. Organization Citrus Virol., IOCV, Univ. Calif., Riverside.
Ruggieri, G. (1955). Le arance impietrate. Riv. Agrumicoltura 1 : 65-69.
Ruggieri, G. (1961). Observations and research on impietratura. p. 182-186. In W. C. Price (Ed.) Proc.2th Conf. Intern. Organization Citrus Virol., Univ. Florida Press, Gainesville.
Russo, F., L.J. Klotz (1963). Tarocco Pit. Calif. Citrograph 48:221-222.
Swingle, W. T., H. J. Webber. (1896). The principle diseases of citrus fruits in Florida. U. S. Dept. Agr. Div. Veg. Phys. Path. Bul. 8: 42pp.
Vernière, C., X. Perrier, C. Dubois, A. Dubois, L. Botella, C. Chabrier, J. M. Bové, N. Duran Vila. (2004). Citrus viroids : symptom expression and effect on vegetative growth and yield of clementine trees grafted on trifoliate orange. Plant Disease 88: 1189-1197.
Vogel, R. (1961). Citrus virus diseases in Corsica. p. 242-244. In W. C. Price (Ed.) Proc.2th Conf. Intern. Organization Citrus Virol., Univ. Florida Press, Gainesville.
Vogel, R., J. M. Bové. (1964). Stem pitting sur bigaradier et sur oranger Tarocco en Corse: une maladie à virus. Fruits 18: 269-274.
Vogel R., J. M. Bové. (1968). Cristacortis, a virus disease inducing stem pitting on sour orange and other citrus species. p. 221228. In J. F. L. Childs (ed.) Proc.4th Conf. Intern. Organization Citrus Virol., Univ. Florida Press, Gainesville.
Vogel R. and J. M. Bove (1972) - Relation of cristacortis virus to other citrus viruses, p. 178-184. In
W.C. Price (ed.) Proc. 5th Conf. Intern. Organization Citrus Virol., Univ. Florida Press, Gainesville.
Vogel R., J. M. Bové. (1974). Studies on the cause of leaf symptoms associated with cristacortis disease of citrus. p. 131-134. In L. G. Weathers, M. Cohen (Eds) Proc. 6th Conf. Intern. Organization Citrus Virol., Univ. Cali., Div. Agr. Sci.
Vogel, R. and J.M. Bove. (1980). Pollen transmission to citrus of the agent inducing cristacortis stem pitting and psorosis young leaf symptoms. p. 188-190. InE. C. Calavan, S. M. Garnsey, and L. W. Tirnmer (eds) Proc. 8th Conf. Intern. Organization Citrus Virol., IOCV, Univ. Calif., Riverside.
Wallace, J. M. (1957). Virus-strain interference in relation to symptoms of psorosis disease of citrus. Hilgardia 27: 223-246.
Wallace, J. M. (1959). A half century of research on psorosis. p. 5-21. In J. M. Wallace (ed.) Citrus virus diseases. Univ. Calif. Div. Agr. Sci., Berkeley, Calif.
Wallace, J.M. (1968). Recent developments in the citrus psorosis diseases, p. 1-9. InJ. F. L. Childs (ed.), Proc. 4th Conf. Intern. Organization Citrus Virol., Univ. Florida Press, Gainesville.
Wallace, J. M. (1978). The psorosis diseases. p. 69-87. In W. Reuther, E. C. Calavan, and G. E. Carman (Eds) The Citrus Industry, vol. IV, Univ. Calif., Div. Agr. Sci.
Prepared (1975) by L. J. Klotz
Department of Plant Pathology
University of California
Riverside, California 92502
(U.S.A.)
Revised April 2009 by C. N. Roistacher1 and J. M. Bové2
1 Department of Plant Pathology
University of California
Riverside, California 92502
(U.S.A.)
2 Laboratoire de Biologie Cellulaire and Moleculaire
I.N.R.A./ Université Victor Ségalen Bordeaux 2,
Centre INRA de Bordeaux,
71, Ave. E. Bourleau,
33883 Villenave d’Ornon,
(FRANCE)
Click any image to see it larger.
PHOTOS |
LEGENDS AND AUTHORS |
|---|---|
|
Bark lesions of psorosis A on trunk and branches of sweet orange trees in California (L. J. Klotz), Corsica (R. Vogel) and Cyprus (J. M. Bové).
|
|
|
Young leaf symptoms of Psorosis A: “flecking” on young clementine leaf (left) and ringspots on mature sweet orange leaf. – J. M. Bové
|
|
|
Mandarin tree on sour orange with severe symptoms of concave gum on scion but no symptoms on rootstock. Nafplion, Greece – J. M. Bové
|
|
|
Severe symptoms of blind pocket on sweet orange trees in Spain (C. N. Roistacher) and Argentina, Salta province (Julia Figueroa).
|
|
|
Upper half: symptoms of crinkly leaf on two Lisbon lemon and two sour orange leaves, California ( P. R. Desjardins). Lower half: symptoms of infectious variegation on Marsh grapefruit leaves, Florida (S. M. Garnsey).
|
|
|
Right: young mandarin leaf with “flecking”, characteristic of psorosis A. (Madagascar) – J. M. Bové Left: young clementine leaf with “flecking” and “oak-leaf pattern”, characteristic not only of concave gum-blindpocket, but also cristacortis and impietratura. (Corsica) – R. Vogel Pale green-filled outline of “oak-leaf pattern” along leaf midvein is represented schematically. |
|
|
Sour orange seedling tree graft-inoculated with cristacortis agent. Left: cristacortis depressions on trunk. Center: removal of bark shows cristacortis depressions to be due to stem pits. Right: cambial side of sour orange bark showing two pegs fitting into cristacortis stem pits. (Corsica) – R. Vogel
|
|
|
Cristacortis young leaf- and wood-symptoms. Left: clementine leaf with cristacortis-induced vein flecking on margins and oak-leaf pattern on each side of leaf midrib. (Corsica) – J. M. Bové Upper right: cross section through cristacortis-affected Orlando tangelo branch. Notice pits and radial traces left by old pits. No gum-stained layers of wood. Lower right: cross section through concave gum-blind pocket-affected Orlando tangelo branch. Notice mild concave gum depressions and fine layers of wood stained with gum. (Corsica) – R. Vogel
|
|
|
Left: sections through Impietratura-affected fruits from a “Double fine” sweet orange tree. Gum pockets in albedo are characteristic of impietratura and are located underneath green spots on the fruit (lower right), Bou Salem, Tunisia – J. M. Bové Upper right: biological indexing for impietratura on grapefruit trees. The inoculum bud (visible within the white circle) was graft-inoculated close to fruits that had just set. Symptoms (gum pockets in albedo) are observed on matured fruits (Corsica) – R. Vogel
|
|
|
Severe concave gum depressions on clementine tree. The trunk and major limbs are totally deformed by multiple, partly overlapping depressions. (Castellón, Spain) – C. N. Roistacher
|
|
|
Severe concave gum depressions and a few blind pocket depressions (BP) on two willow-leaf mandarin trees. Symptoms affect the scion trunk and branches, but not the tolerant sour orange rootstock. (Corsica) – J. M. Bové
|
|
|
Two views of a Sampson tangelo tree on sour orange with severe concave gum symptoms on scion (gnarled trunk and limbs, broad depressions) but no symptoms on tolerant rootstock. (Algeria) – J. M. Bové
|
|
|
Two Orlando tangelo trees on sour orange with severe concave gum symptoms on scions but no symptoms on tolerant sour orange rootstocks. (Corsica) – J. M. Bové
|
|
|
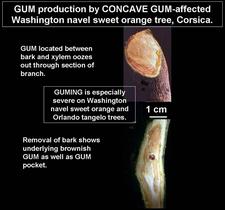
Gumming on a small branch of a Washington navel sweet orange tree affected by concave gum. R. Vogel
|
|
|
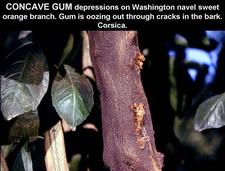
Early concave gum depressions and gumming on small branch of a Washington navel sweet orange tree. Gum is oozing out through cracks in the bark. R. Vogel
|
|
|
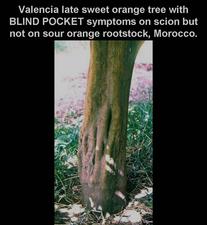
Valencia late sweet orange tree on sour orange with deep, narrow depressions of blind pocket on scion trunk but no symptoms on tolerant rootstock. (Morocco) – J. M. Bové
|
|
|
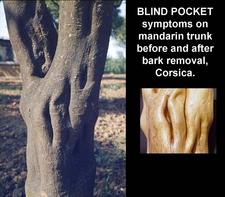
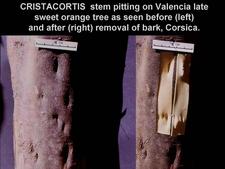
Blind pocket depressions on willow leaf mandarin tree before (left) and after (right) removal of bark. (Corsica) – J. M. Bové
|
|
|
Cross section through a limb showing one blind pocket depression (old, narrow and deep) and two concave gum depressions (early and broad). (California) – T. A. Dewolfe
|
|
|
Willow leaf mandarin trunks after removal of bark showing blind pocket depressions (left) compared to concave gum depressions (right). Trunk on right shows part of the scion, with severe depressions, and part of the tolerant sour orange rootstock, the tissues of which, below the budunion line, are deformed by the depressions on the sensitive mandarin scion above the budunion line. (Corsica) – R. Vogel
|
|
|
Comparison of various concave gum-blind pocket depressions. A: three typical concave gum (CG) depressions on willow leaf mandarin. B: willow leaf mandarin depression half way between CG depression and blind pocket depression. C: typical blind pocket (BP) depression on sweet orange. (Corsica – J. M. Bové
|
|
|
Cross sections through a young Washington navel sweet orange branch. The sections show concentric wood layers partly stained with gum and characteristic of concave gum-blind pocket, especially on Washington navel sweet orange and Orlando tangelo. (Corsica) – R. Vogel
|
|
|
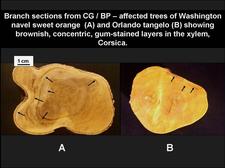
Brownish, concentric, gum-stained wood layers, characteristic of concave gum-blind pocket, on section from a Washington navel sweet orange limb (A) and a Orlando tangelo branch (B). (Corsica) – R. Vogel
|
|
|
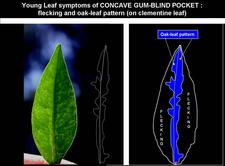
Young leaf symptoms of concave gum-blind pocket: flecking and aok-leaf pattern, shown here on Clementine leaf. The oak-leaf pattern extends on each side of the leaf midrib and is shown in blue on the leaf sketch at right. Flecking is between leaf margins and oak-leaf pattern as written on the sketch. R. Vogel and J.M. Bové
|
|
|
Concave gum-blind pocket induced oak-leaf pattern on young Satsuma leaf (Corsica) and sweet orange leaf (Morocco). Outline of oak-leaf pattern is shown by white line. J. M. Bové
|
|
|
Hamlin (left) and Vernia (right) sweet orange trees on sour orange rootstocks showing cristacortis depressions. These vertical depressions are shorter than blind pocket depressions. Cristacortis depressions are seen not only on sensitive scions, here sweet orange, but characteristically also on the sensitive sour orange rootstock. Concave gum-blind pocket is never seen on sour orange, a rootstock tolerant to concave gum-blind pocket. The red knife sticking into the tree indicates the budunion line. (Corsica) – R. Vogel
|
|
|
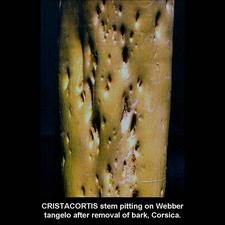
Removal of bark shows cristacortis depressions to be associated with stem pits, as seen here on a severely affected Webber tangelo seedling inoculated with the cristacortis agent. (Corsica) – R. Vogel
|
|
|
Left. Cristacortis depressions on trunk of Valencia sweet orange tree before removal of bark. Right. Bark removal shows: (i) pits underlying the depressions, and (ii) presence of pegs on the cambial side of the removed patch of bark, and these pegs fit into the corresponding pits in the wood. No such pegs and pits are observed with concave gum-blind pocket depressions. On old, cristacortis-affected trees, stem pitting may be seen not only on trunk and limbs, but also on young branches, 1cm in diameter. Concave gum-blind pocket depressions appear not as early as those of cristacortis. (Corsica) – R. Vogel
|
|
|
Comparison between wood symptoms of concave gum-blind pocket and wood symptoms of cristacortis. Left:: cross section through concave gum-blind pocket-affected Orlando tangelo branch. Notice mild concave gum depressions and fine layers of xylem (wood) stained with gum. Corsica. These concentric, gum-stained wood layers are characteristic of concave gum-blind pocket. Right: cross section through cristacortis-affected Orlando tangelo branch. Notice pits and radial traces left by old pits. These pits and traces are characteristic of cristacortis, and there are no gum-stained layers of wood. (Corsica) – R. Vogel. See also CG-BP. 08 and CG-BP. 29
|
|
|
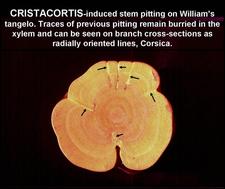
Wood symptoms of cristacortis on cross section of a William’s tangelo branch. The arrows point at traces of old pits. These traces are buried in the xylem and can be seen on the cross section as radially oriented lines. The pits and traces are characteristic of cristacortis and are not seen with concave gum-blind pocket. (Corsica) – R. Vogel
|
|
|
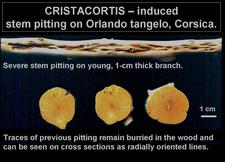
On Orlando tangelo seedlings graft-inoculated with the cristacortis agent, severe stem pitting may develop on very young branches, only ~1cm in diameter, and the radially oriented traces of old pits can be seen on cross sections of branches only a few centimetres in diameter. (Corsica) – R. Vogel
|
|
|
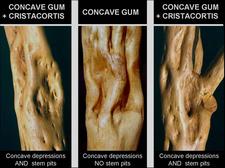
Comparison between concave gum-Blind pocket depressions and stem pitting symptoms of cristacortis on trunks of Orlando tangelo seedling trees after removal of bark. Center: trunk of Orlando tangelo seedling tree graft-inoculated with the concave gum-blind pocket agent alone, and showing broad concave depressions. Right and left: trunks of Orlando tangelo seedling trees graft-inoculated with the two disease agents. In addition to the broad concave depressions, the trunks show the many cristacortis stem pits, much smaller than the concave gum-blind pocket depressions. (Corsica) – R. Vogel
|
|
|
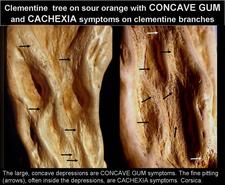
Comparison between concave gum-Blind pocket depressions and stem pitting symptoms of cachexia on trunks of Clementine trees on sour orange after removal of bark. The broad depressions are those of concave gum-blind pocket. The fine stem pitting (arrows), often within the concave depressions, is caused by the cachexia viroid. (Corsica) – R. Vogel
|
|
|
Symptoms of “tarocco pit” on Tarocco sweet orange tree after removal of bark. Pits are filled with brownish, cork-like material. (Sicily) – J. M. Bové
|
|
|
Willow leaf mandarin branch showing “finger marks”. Such marks are probably not transmissible by graft-inoculation. (Spain) – J. Gil-Fibla
|
|
|
Flecking and oak-leaf patterns induced by the concave gum-blind pocket agent on young Dweet tangor leaves. (California) – C. N. Roistacher
|
|
|
Oak-leaf patterns on leaves of indicator plants of Dweet tangor, sweet orange or mandarin are diagnostic for concave gum-blind pocket, cristacortis or impietratura. (California) – C. N. Roistacher
|
|
|
Oak- leaf patterns on leaves of a Symons sweet orange seedling graft-inoculated with budwood from a Minneola tangelo, which showed characteristic concavities of concave gum. (Australia) – Patricia Barkley
|
|
|
Early symptoms of concave gum-blind pocket depressions on young branches of a Washington navel sweet orange tree. (Corsica) – R. Vogel.
|
|
|
The first symptoms to appear on the young emerging flush of seedlings of sweet orange, mandarin or Dweet tangor, within 5 to 8 weeks after graft-inoculation of the agent of concave gum-blind pocket, but also cristacortis and impietratura, is interveinal flecking as shown in the two leaves on the left. There are usually no oak leaf patterns in this initial symptom. The un-inoculated control leaf is on the right. C. N. Roistacher
|
|
|
LEFT. Shock symptoms on sweet orange seedling graft-inoculated with Psorosis A virus. Shock symptoms (wilting, bending and decline) are observed of the first young shoots developing after inoculation and cutting back the index seedling. RIGHT. No shock symptoms develop on sweet orange seedling graft-inoculated with the agent of concave gum-blind pocket, cristacortis or impietratura. C. N. Roistacher
|