Citrus Tatter Leaf
HISTORY, DISTRIBUTION AND IMPORTANCE
Citrus tatter leaf was first described in California in 1962 by Wallace & Drake (1962) who reported that leaves of Citrus excelsa graft-inoculated with tissue from a symptomless Meyer lemon developed yellow blotches and edge malformation. They later reported that citrange and citremon also developed marked leaf and stem effects when inoculated with tatter leaf-infected Meyer lemon (Wallace & Drake, 1963). They then found that subsequent new growth of C. excelsa was symptomless, but if re-inoculated, symptoms developed again on new growth – also, if the symptomless tissue was used to graft-inoculate citrange, leaf and stem symptoms developed (Wallace & Drake, 1968). They concluded that there were two viruses in the original Meyer lemon, tatter leaf (causing the C. excelsa symptoms) and citrange stunt (causing the citrange and citremon symptoms). Roistacher et al. (2000) carried out a 22-year long series of trials to try and separate the two proposed viruses by sequential passaging through a variety of citrus hosts, but were unable to achieve separation, concluding that only one virus was involved. They suggested adoption of the name ‘tatter leaf’.
Tatter leaf has been reported from California, Florida and Texas in the USA, Japan, China, Taiwan, Korea, Brazil, Taiwan, Thailand, Philippines, Australia and South Africa. It has been associated with serious bud union incompatibility in trees on trifoliate and trifoliate hybrid rootstocks in Japan (Miyakawa & Matsui, 1988), China (Ke & Wu, 1988; Zhang et al., 1988) and South Africa (Marais & Lee, 1986). The virus has become potentially more important because Citrus tristeza virus is spreading into new regions requiring a switch to CTV tolerant rootstocks including those, which are Citrus tatter leaf virus (CTLV)-sensitive.
NAME OF DISEASE AND SYNONYMS
The original name given was Tatter leaf disease because of the deformed leaf symptoms induced in C. excelsa. Citrange stunt was first reported in 1968 as a new virus causing symptoms on citrange, but since it was eventually established that the two were one and the same, the original name was preserved.
The virus causes bud-union crease in trees on trifoliate orange rootstock (Miyakawa & Matsui, 1988).
Abbreviation : TL
SYMPTOMOLOGY
General aspect of affected field tree
Trees on susceptible rootstocks (trifoliate orange and hybrids) can appear stunted and chlorotic (TL. 01).
Symptoms on trunk (on rootstock and/or scion), limbs and shoots
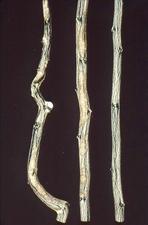
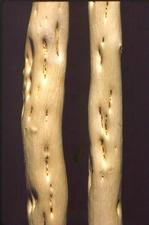
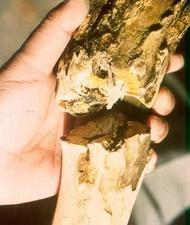
Stems of citranges can be deformed and have a ‘zig-zag’ growth pattern (TL. 02) and develop blotches (TL. 03). Citremon develops pitting in the wood (TL. 04). Trees on trifoliate orange or trifoliate hybrid rootstocks have fluted growth of the rootstock (TL. 05) and can develop a bud-union crease (TL. 06). In strong winds, scions have been known to snap off at the bud union (TL. 07).
Symptoms on leaves
Infected C. excelsa leaves have torn or ragged edges (TL. 08), and citrange leaves develop chlorotic spots, mottling and distortion (TL. 09). Ringspots have also been observed on infected citrumelo leaves (TL. 10).
Symptoms on fruit
A distortion of fruit rind (intumescence) in Cleopatra mandarin has been associated with the virus (Lovisolo et al., 2003) (TL. 11).
Histological and cytological symptoms
Crystallized virus bodies have been observed in phloem cells of citrange and mandarin, and mesophyll cells of Chenopodium quinoa and Nicotiana clevelandii (Chen et al., 1995). The chloroplasts of these mesophyll cells had accumulated starch bodies, and the lamella was degraded. Virus particles have been observed in cells as banded aggregates (Inoue et al., 1979).
CAUSAL AGENT: DESCRIPTION AND PROPERTIES
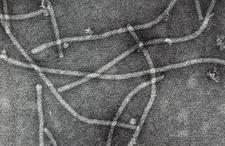
Semancik and Weathers (1965) purified rod shaped particles (650 x 19 nm) from cowpeas mechanically inoculated with tatter leaf (see host range). Later electron microscopy showed the particles to have criss-cross (TL. 12) or cross banding patterns (TL. 13). The CTLV genomic RNA has been cloned and sequenced, and shown to be indistinguishable from Apple stem grooving virus (ASGV), type member of the Capillovirus genus in the family Flexiviridae (Ohira et al., 1995; Yoshikawa et al., 1993).
There may be some strain differences. In Texas nine out of 16 isolates caused bud union disorders, and there were some different reactions using two ASGV monoclonal antibodies (Herron & Skaria, 2000).
HOST RANGE
Sensitive species, varieties or combinations
Chlorotic leaf symptoms are produced in Mexican lime, C. excelsa, citranges and other trifoliate orange hybrids. C. excelsa leaves can initially be deformed (hence the name ‘tatter leaf’), but infected plants often recover and remain symptomless. Citrange, citremon and citrumelo stems develop a ‘zig-zag’ growth pattern. The virus is irregularly distributed in citranges, and often cannot be recovered from symptomless areas.
CTLV can be mechanically transmitted to many herbaceous hosts. In one study, 19 species of nine families were infected (Semancik & Weathers, 1965). Cowpea (Vigna sinensis cv. Early Ramshorn) shows diffuse necrotic lesions 3-4 days after inoculation (TL. 14). Chenopodium quinoa and C. amaranticolor deveop local lesions and systemic mottle (TL. 15). Ipomea purpurea and Phaseolus vulgaris show necrotic local lesions. Nicotiana clevelandii developed etched ringspots on inoculated leaves and a systemic mottle and vein clearing, while N. glutinosa had only these systemic symptoms. CTLV has been isolated from wild lily with yellowing symptoms in Japan (Inouye et al., 1979) (TL. 16, 17).
Symptomless species, varieties or combinations
- Immune: trifoliate orange
- Tolerant (symptomless carriers): Sweet orange, sour orange, grapefruit, lemon and mandarin are among the latent citrus hosts, although Cleopatra mandarin may develop fruit symptoms. Other plants such as Catharanthus roseus (periwinkle), Petunia axillaris, Zinnia elegans, Pisum sativum, Glycine max and Crotalaria spectabilis are symptomless hosts.
TRANSMISSION
Natural
Cultural transmission through budwood and mechanical transmission.
Transmission by cutting or pruning tools may have occurred in Texas from a Meyer lemon tree to neighboring Eureka lemon and Cleopatra mandarin trees (da Graça & Skaria, 1996).
No evidence for seed or vector transmission
Experimental
Graft inoculation with shoots, buds. For reactive species, grafting of symptomatic tissues is necessary.
Mechanical transmission
EPIDEMIOLOGY
Not studied
DIAGNOSIS
Diagnostic field symptoms
Bud union crease in trees on trifoliate orange and trifoliate hybrid rootstocks.
Biological indexing
Biological indexing by graft inoculating Rusk or Troyer citrange in a cool greenhouse (Roistacher, 1991), and watching for symptom development, which should appear in 1-6 months. Rusk citrange budded on rough lemon rootstock develops good symptoms at warmer temperatures, and is being used in Florida and California (R. F. Lee, pers. comm.).
Serological and molecular techniques
ELISA kits using both polyclonal and monoclonal antibodies have been developed, as have PCR-based techniques (Hailstones et al., 2000). The latter have been shown to be effective in multiplex assays with several viroids (Ito et al., 2002).
CONTROL
Use of virus-free budwood. Production of virus-free material is difficult because the virus is difficult to eliminate through shoot-tip grafting, although pre-heat treatment can improve success (Koizumi, 1984); thermotherapy on its own is also effective (Roistacher et al., 1972). Since it is mechanically transmissible, cutting and pruning tools need to be sterilized with sodium hypochlorite.
SELECTED REFERENCES
Chen, J., T. Zhang, X. Zhou, D. Li (1995). A study on citrus tatter leaf virus. Acta Microbiol. Sin. 35: 173-180.
da Graça, J. V., M. Skaria (1996). Citrus tatter leaf virus in the Rio Grande Valley of south Texas. p. 198-200 In J. V. da Graça, P. Moreno, R. K. Yokomi (eds.) Proc. 13th Conf. Intern. Organization Citrus Virol. IOCV, Riverside, CA
Hailstones, D. L., K. L. Bryant, P. Broadbent, C. Zhou (2000). Detection of Citrus tatter leaf virus with reverse transcription-polymerase chain reaction. Aust. Plant Pathol. 29: 240-248.
Herron, C. M., M. Skaria (1996). Further studies on Citrus tatter leaf virus in Texas. p. 185-194 In J. V. da Graça, R. F. Lee, R. K. Yokomi (eds.) Proc. 14th Conf. Intern. Organization Citrus Virol. IOCV, Riverside, CA.
Inouye, N., T. Maeda, K. Mitsuhata (1979). Citrus tatter leaf virus isolated from lily. Ann. Phytopathol. Soc. Jap. 54: 712-720.
Ito, T., H. Ieki, K. Ozaki (2002). Simultaneous detection of six citrus viroids and Apple stem grooving virus from citrus plants by multiplex reverse transcription polymerase chain reaction. J. Virol. Methods 106: 235-239.
Ke, C., R. Wu (1991). Occurrence and distribution of Citrus tatter leaf in Fujian, China. p. 358-364 In R. H. Brlansky, R. F. Lee, L. W. Timmer (eds.) Proc. 11th Conf. Intern. Organization Citrus Virol., IOCV, Riverside, CA
Koizumi, M. (1984). Elimination of tatter leaf-citrange stunt virus from Satsuma mandarin by shoot-tip grafting following preheat treatment. p. 229-233 In S. M. Garnsey, L. W. Timmer, J. A. Dodds (eds.) Proc. 9th Conf. Intern. Organization Citrus Virol. IOCVC, Riverside, CA.
Lovisolo, O., G. P. Accotto, V. Masenga, A. Colariccio (2003). An isolate of Apple stem grooving virus associated with Cleopatra mandarin fruit intumescence. Fitopatol. Bras. 28: 54-58.
Marais, L. J., R. F. Lee (1986). Citrange stunt virus associated with decline of Shamouti on Swingle rootstock in South Africa. Plant Dis. 70: 892.
Miyakawa, T., C. Matsui (1988). The association of tatter leaf virus with budunion crease of trees on trifoliate orange rootstock. p. 360-364 In L. W. Timmer, S. M. Garnsey, L. Navarro (eds.) Proc. 10th Conf. Intern. Organization Citrus Virol. IOCV, Riverside, CA
Ohira, K., S. Nimba, M. Rosanov, T. Kusumi & T. Tsuchizaki (1995). Complete sequence of an infectious full length cDNA clone of citrus tatter leaf capillovirus: comparative sequence analysis of capillovirus genomes. J. Gen. Virol. 76: 2305-2309.
Roistacher, C. N. (1991). Tatterleaf. p. 99-103 In C. N. Roistacher (ed.) Graft-transmissible Diseases of Citrus. Handbook for Detection and Diagnosis. FAO, Rome.
Roistacher, C. N., J. Bash, D. J. Gumpf (2000). Continued attempts over a 22-year period to separate components of the citrus tatter leaf-citrange stunt virus complex. In p. 179-184 In J. V. da Graça, R. F. Lee, R. K. Yokomi (eds.) Proc. 14th Conf. Intern. Organization Citrus Virol. IOCV, Riverside, CA.
Roistacher, C. N., E. C. Calavan, E. M. Nauer, W. Reuther (1972). Virus-free Meyer lemon trees. Citrograph 57: 250, 270-271.
Semancik, J. S., L. Weathers (1965). Partial purification of a mechanically transmissible virus associated with tatter leaf of citrus. Phytopathology 55: 1354-1358.
Wallace, J. M., R. J. Drake (1962). Tatter leaf, a previously undescribed virus effect on citrus. Plant Dis. Reptr. 46: 211-212.
Wallace, J. M., R. J. Drake (1963). New information on symptom effects and host range of citrus tatter leaf virus. Plant Dis. Reptr. 46: 352-353.
Wallace, J. M., R. J. Drake (1968). Citrange stunt and ringspot, two previously undescribed virus diseases of citrus. p. 177-180 In J. F. L. Childs (ed.) Proc. 4th Conf. Intern. Organization Citrus Virol. Univ. Florida Press, Gainesville, FL.
Yoshikawa, N. M. Imaizumi, T. Takahashi, N. Inouye (1993). Striking similarities between the nucleotide sequence and genome organization of citrus tatter leaf virus and apple stem grooving capilloviruses. J. Gen Virol. 74: 2743-2747.
Zhang, T. M., X. Y. Liang, C. N. Roistacher (1988). Occurrence and detection of citrus tatter leaf virus (CTLV) in Huangyan, Zhejiang Province, China. Plant Dis. 72: 543-545.
“Tatter leaf” and “Citrange stunt” prepared (1975, 1981) by J. M. Wallace
University of California
Riverside CA 92521
(USA)
and
“Tatter leaf – budunion disorder” prepared by J. M. Bové (1981)
Université de Bordeaux et INRA
Pont de la Maye
(France)
Revised (2008) by J. V. da Graça
Texas A & M University-Kingsville
Citrus Center
Weslaco TX 78596
(USA)
Click any image to see it larger.
PHOTOS |
LEGENDS AND AUTHORS |
|---|---|
|
Retarded growth of a 3-yr old navel sweet orange on Troyer citrange citrange experimentally infected with CTLV before being planted in the field (California, USA) – J. M. Wallace
|
|
|
Zig-zag stem growth of Troyer citrange caused by CTLV (California) – J. M. Wallace
|
|
|
Bark blotches on CTLV-infected Troyer citrange (healthy stem on left) (California, USA) – J. M. Wallace
|
|
|
CTLV stem-pitting on citremon (California, USA) – J. M. Wallace
|
|
|
Fluting of Troyer citrange rootstock caised by CTLV (same tree as in TL-01) (California, USA) – J. M. Wallace
|
|
|
Satsuma mandarin on Troyer citrange with bark removed to show clear bud union crease and fluting of the rootstock (California, USA) – E. C. Calavan
|
|
|
Presence of CTLV can result in breakage at the bud union during wind storms as shown in this mandarin on trifoliate orange (Japan) – C. N. Roistacher
|
|
|
Leaves of CTLV-infected Citrus excelsa with irregularly indented margins and varying degrees of mottling. Some leaves remain small. Healthy leaf on the left. (California, USA) – J. M. Wallace
|
|
|
Leaves of CTLV-infected Troyer citrange with mottling and distortion. Healthy leaf on the left. (California, USA) – J. M. Wallace
|
|
|
Ringspots on citrumelo (Texas) - M. Skaria
|
|
|
Cleopatra mandarin fruit from a CTLV-infected tree displaying intumescent areas (São Paulo, Brazil) – A. Colariccio
|
|
|
Purified CTLV particles with criss cross pattern (Japan) – T. Nishio et al.
|
|
|
Purified CTLV particles with cross banding pattern (Japan) – T. Nishio et al.
|
|
|
Healthy cowpea leaf (left) and two leaves with reddish necrotic local lesions as a result of mechanical inoculation with CTLV. (California, USA) – L. G. Weathers
|
|
|
Systemic severe distortion and mottle on leaves Chenopodium quinoa mechanically inoculated with CTLV (California, USA) – C. N. Roistacher
|
|
|
Lily with stunting symptoms caused by CTLV (Japan) – N. Inouye
|
|
|
Leaf yellowing and streaking in lily caused by CTLV (Japan) – N. Inouye
|